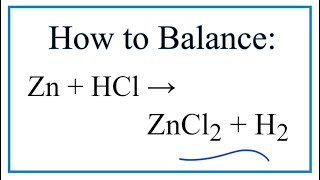
Zinc plus hydrochloric acid
Wiki User. A word equation represent the reactions between metals and acids. The reaction for zinc and hydrochloric acid would be, zinc plus hydrochloric acid produces hydrogen plus zinc chloride. Matter can neither be created nor destroyed.
Wiki User. A word equation represent the reactions between metals and acids. The reaction for zinc and hydrochloric acid would be, zinc plus hydrochloric acid produces hydrogen plus zinc chloride. Zinc plus hydrochloric acid produces zinc chloride plus hydrogen gas. Common one. Cl H Zn -apex Fojus xD. Approximately g of hydrogen gas are produced when
Zinc plus hydrochloric acid
.
Zinc chloride and hydrogen gas. All Rights Reserved. Nitric acid plus zinc oxide?
.
We can use a gas syringe to measure the reaction of metals with dilute acid. When zinc reacts with hydrochloric acid it produces zinc chloride and hydrogen gas. We can measure the rate of the reaction by measuring how fast the reaction produces hydrogen. This requires a conical flask and gas syringe. Similarly, when calcium carbonate reacts with dilute hydrochloric acid, it produces carbon dioxide gas. We can measure the rate of the reaction by measuring how fast the reaction produces carbon dioxide. Hydrogen peroxide decomposes in the presence of a catalyst close catalyst A substance that increases the rate of a chemical reaction without being used up. We can measure how each catalyst affects the rate of the reaction by measuring how fast it produces oxygen. The same apparatus is used — alternatively you can replace the gas syringe with a measuring cylinder filled with water and inverted in a trough of water. Sodium thiosulfate reacts with dilute hydrochloric acid to produce sodium chloride, water, sulfur dioxide and sulfur.
Zinc plus hydrochloric acid
It is fairly obvious that zinc metal reacts with aqueous hydrochloric acid! The bubbles are hydrogen gas. When zinc metal is submerged into a quantity of aqueous HCl, the following reaction occurs Figure 5. This is one example of what is sometimes called a single replacement reaction because Zn replaces H in combination with Cl. Because some of the substances in this reaction are aqueous, we can separate them into ions:.
Eva lovia pov
What is the equation for zinc plus sulfuric acid? If it isn't, then sorry. Distillation, evaporation, filtration or neutralization? Still have questions? Related questions. Related questions. What is the word equation for hydrochloric acid and zinc carbonate? Find more answers. A word equation represent the reactions between metals and acids. The products are zinc chloride and hydrogen.
The hydrogen causes bubbling during the reaction, and can be detected using a burning splint which produces a squeaky pop sound. In general, the more reactive the metal, the faster the reaction. This is indicated by more bubbles being given off per second from the metals with higher reactivity, as shown by this diagram.
Log in. Continue Learning about Chemistry. Still have questions? Distillation, evaporation, filtration or neutralization? Best Answer. More answers. Resources Leaderboard All Tags Unanswered. Chemical equation for reaction between zinc and hydrocloric acid? The products are zinc chloride and hydrogen. Approximately g of hydrogen gas are produced when The hydrochloric acid isn't destroyed, only changed into something else. Trending Questions. What is the equation for the dissociation of hydrochloric acid? Zinc chloride and hydrogen gas are formed in the following reaction. What is the symbol equation for zinc plus hydrochloric acid zinc plus chloride hydrogen?

It is remarkable, rather amusing phrase
In it something is. I thank for the information, now I will know.
I can not take part now in discussion - it is very occupied. But I will soon necessarily write that I think.