Xeo2f2 lewis structure
N will be equal to the number of monovalent fluorine atoms bonded to the central atom. C will be the charge of cation and A will be the charge of xeo2f2 lewis structure.
In order to find the total valence electrons in XeO2F2 molecule , first of all you should know the valence electrons present in xenon atom, oxygen atom as well as fluorine atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Xenon is a group 18 element on the periodic table. Oxygen is group 16 element on the periodic table. Fluorine is group 17 element on the periodic table. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center.
Xeo2f2 lewis structure
Transcript: This is Dr. Let's do the XeO2F2 Lewis structure. For XeO2F2, we have a total of 34 valence electrons. We'll put the Xe in the center and then we'll put an Oxygen here and here, and a Fluorine right there and there. We'll put a chemical bond right here between the atoms. Each bond represents two electrons, so we've used 2, 4, 6, 8 valence electrons. And then we'll fill the octets of the outer shell. So we have 8, 10, 12, 32, and remember we had So we've used 32 and we'll put those remaining two right here on the central Xenon atom. When you check the formal charges, you'll find out that it looks just like this. So while all the charges add up to zero, overall we'd like to have everything, each of the atoms, at zero itself. So let's try another structure and see how that looks. That should get rid of part of it.
We're still using 34 valence electrons, so that's OK. Each bond represents two electrons, so we've used 2, 4, 6, 8 valence electrons.
Laurence Lavelle Skip to content. Quick links. Email Link. XeO2F2 Lewis Structure Post by Bryce Ramirez 1J » Wed Nov 20, am Why does Xenon have two double bonds with oxygen and 2 single bonds with fluorine and a lone pair to make the lowest energy level for its lewis structure. I thought that you are only allowed to assign 8 electrons to each atom, but if you were to add it up, Xe would have 14 electrons.
In this article, we will discuss about xeo2f2 lewis structure, hybridization, formal charge, and its geometry. Xenon dioxide difluoride, sometimes known as XeO 2 F 2 , is an inorganic molecule with the chemical formula XeO 2 F 2. One xenon atom, two oxygen atoms, and two fluorine atoms make up XeO 2 F 2 xenon dioxydifluoride. Two single bonds and two double bonds surround the xenon atom in the Lewis structure of XeO2F2, which is surrounded by two fluorine atoms and two oxygen atoms. There are three lone pairs in each fluorine atom, two lone pairs in each oxygen atom, and one lone pair in each xenon atom. The Lewis structure of an atom is a simplified depiction of the nucleus and valence electrons in its atomic structure. It depicts the electron configuration in an atom.
Xeo2f2 lewis structure
Ready to learn how to draw the lewis structure of XeO2F2? Here, I have explained 5 simple steps to draw the lewis dot structure of XeO2F2 along with images. The Xenon atom has 1 lone pair. Note: Take a pen and paper with you and try to draw this lewis structure along with me.
To allow traduction
The shape of XeO 2 F 2 is a see-saw. Valence electrons are the electrons that are present in the outermost orbit of any atom. He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations. Xenon is a group 18 element on the periodic table. Transcript: This is Dr. By doing so, you will get the following lewis structure of XeO2F2. Who is online Users browsing this forum: No registered users and 3 guests. Thus, we can put those electrons in one of the d-orbitals. If we compare the electronegativity values of xenon Xe , oxygen O and fluorine F then the xenon atom is less electronegative. So let's try another structure and see how that looks. This is an exception to the octet rule wherein the octet expands to hold more than 8 electrons. About author.
Xenon dioxide difluoride is an inorganic compound denoted by the chemical formula XeO 2 F 2. It has a molecular weight of
The fluorine atom will be the monovalent surrounding atom and the oxygen atom will be the divalent surrounding atom. Transcript: This is Dr. In Xenon Dioxide Difluoride, there will be 5 sp 3 d hybrid orbitals. Also, in step 1 we have calculated the total number of valence electrons present in the XeO2F2 molecule. I thought that you are only allowed to assign 8 electrons to each atom, but if you were to add it up, Xe would have 14 electrons. Here, fluorine will be axial atoms and oxygen will be equatorial atoms. Scroll to Top. Put your understanding of this concept to test by answering a few MCQs. And this is a more stable lewis structure. That should get rid of part of it.

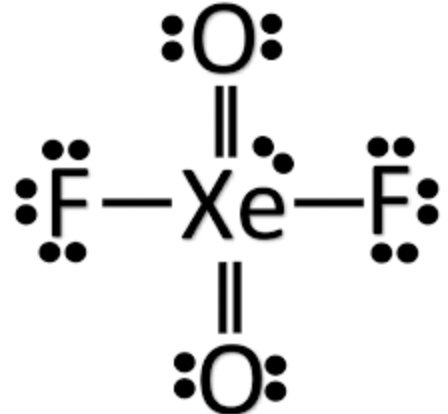
Very useful idea
It is removed
I consider, what is it � a false way.