Sf4 bond angle
The process of mixing of atomic orbitals belonging to the same atom of slightly different energies so that a redistribution of energy takes place between them resulting in the formation of new sets of orbitals of equivalent energies and shape is called hybridization. The new orbitals in this form are known as hybrid orbitals. Like pure orbitals the hybrid orbitals are used in Bond formation. Hybridization is a hypothetical concept and has been introduced in order to explain sf4 bond angle characteristic geometrical shapes of polyatomic molecules, sf4 bond angle.
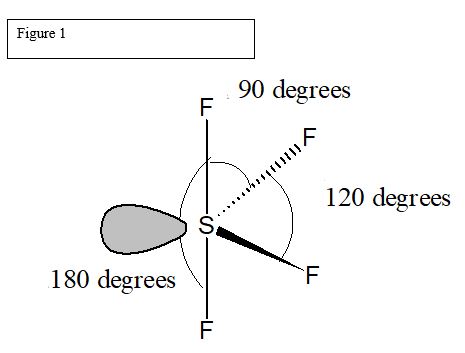
Let us learn about the SF4 molecular geometry and bond angles. You will also get to know more about SF4 structure, SF4 hybridisation, lewis structure of SF4, and the importance of SF4 molecular geometry and bond angles. The structure of SF4 molecular geometry may be predicted using VSEPR theory principles: A nonbonding lone pair of electrons occupy one of the three equatorial locations. As a result, there are two types of F ligands in the molecule: axial and equatorial. The SF4 molecular geometry and bond angles of molecules having the chemical formula AX4E are trigonal bipyramidal. The equatorial orientations of two fluorine atoms establishing bonds with the sulphur atom are shown, while the axial locations of the other two are shown.
Sf4 bond angle
The hybridization that is involved in SF 4 is sp 3 d type. Here will learn and understand how to determine SF 4 hybridization. We will discuss the steps in detail. In order to determine the hybridization of sulphur tetrafluoride, you have to first understand its Lewis structure and the number of valence electrons that are present. The SF 4 molecule consists of a total of 34 valence electrons. Here 6 will come from sulphur and each of the four fluorine atoms will have 7 electrons. During the formation of SF4, the sulphur atom will form bonds with each of fluorine atoms where 8 of valence electrons are used. Meanwhile, the four fluorine atoms will have 3 lone pairs of electrons in its octet which will further utilize 24 valence electrons. In addition, two electrons will be kept as lone pair in the sulphur atom. When bonding takes place there is a formation of 4 single bonds in sulphur and it has 1 lone pair.
Like pure orbitals the hybrid orbitals are used in Bond formation.
What is the shape of SF 4 including bond angles? The formula used to calculate the hybridization of a molecule is as follows:. V is the number of valence electrons present in the central atom. N is the number of monovalent atoms bonded to the central atom. C is the charge of cation.
One needs to know some basic properties of the given compound and its Lewis structure to understand its molecular geometry, polarity, and other such properties. SF4 is a chemical formula for Sulfur Tetrafluoride. It is a colorless corrosive gas that is used in the synthesis of several organofluorine compounds. SF4 is a rather hazardous compound but is used widely in chemical and pharmaceutical companies. It is easy to understand the molecular geometry of a given molecule by using the molecular formula or VSEPR model.
Sf4 bond angle
The Lewis electron-pair approach can be used to predict the number and types of bonds between the atoms in a substance, and it indicates which atoms have lone pairs of electrons. This approach gives no information about the actual arrangement of atoms in space, however. Keep in mind, however, that the VSEPR model, like any model, is a limited representation of reality; the model provides no information about bond lengths or the presence of multiple bonds. The VSEPR model can predict the structure of nearly any molecule or polyatomic ion in which the central atom is a nonmetal, as well as the structures of many molecules and polyatomic ions with a central metal atom. The premise of the VSEPR theory is that electron pairs located in bonds and lone pairs repel each other and will therefore adopt the geometry that places electron pairs as far apart from each other as possible. This theory is very simplistic and does not account for the subtleties of orbital interactions that influence molecular shapes; however, the simple VSEPR counting procedure accurately predicts the three-dimensional structures of a large number of compounds, which cannot be predicted using the Lewis electron-pair approach. We can use the VSEPR model to predict the geometry of most polyatomic molecules and ions by focusing only on the number of electron pairs around the central atom , ignoring all other valence electrons present. According to this model, valence electrons in the Lewis structure form groups , which may consist of a single bond, a double bond, a triple bond, a lone pair of electrons, or even a single unpaired electron, which in the VSEPR model is counted as a lone pair. Because electrons repel each other electrostatically, the most stable arrangement of electron groups i.
Anime furry porn
The form will be equatorial since the lone pair is in the equatorial plane. In SF4, how many lone pairs of electrons are there on the S atom? Work Experiences. Campus Experiences. Question bf. Sulphur is a periodic table group VIA element with six electrons in its final shell valence shell. Related questions How do I determine the bond angle in a molecule? What is the exact bond angle and shape of hypochlorous acid and hypochlorite ion. Current difficulty :. The hybridization of SF 4 is sp 3 d. Byju's Answer. Suggest Changes. Partially ionic links are referred to as polar covalent bonds.
Thionyl tetrafluoride , also known as sulfur tetrafluoride oxide , is an inorganic compound with the formula S O F 4. It is a colorless gas. The shape of the molecule is a distorted trigonal bipyramid, with the oxygen found on the equator.
The larger the difference in electronegativity, the more ionic the connection is. Ans : Not all ligands peripheral groups in trigonal bipyramidal molecules or complexes are equidi Molecular Orbital Theory. Types of Impurity Defects. JEE Examination Scheme. You can put sulfur in the middle because fluorine tends to make single bonds. Image will be Updated soon. The electron pairs will be organised as a trigonal bipyramid, with the lone pair in the centre. Let us learn about the SF4 molecular geometry and bond angles. Learn more topics related to Chemistry. Similar Reads. Share your suggestions to enhance the article. Hybridization is a hypothetical concept and has been introduced in order to explain the characteristic geometrical shapes of polyatomic molecules. Skip to content. Why is molecular geometry important?

It do not agree
The theme is interesting, I will take part in discussion. I know, that together we can come to a right answer.
I consider, that you are not right. I am assured. Let's discuss it. Write to me in PM, we will talk.