Piperdine
Molfile expand, piperdine. A molecular entity having an available pair of electrons capable of forming a covalent bond with a hydron Br o nsted base or with the vacant orbital of some other molecular entity Lewis base. A substance that increases the rate of a reaction without modifying the overall piperdine Gibbs energy change in the reaction, piperdine.
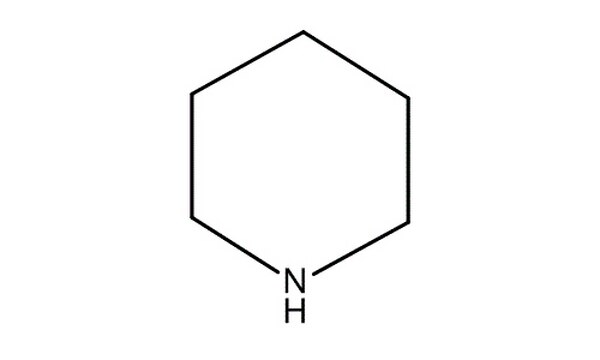
Piperidine is an organic compound with the molecular formula CH 2 5 NH. This heterocyclic amine consists of a six-membered ring containing five methylene bridges —CH 2 — and one amine bridge —NH—. It is a colorless liquid with an odor described as objectionable, typical of amines. Piperidine was first reported in by the Scottish chemist Thomas Anderson and again, independently, in by the French chemist Auguste Cahours , who named it. Industrially, piperidine is produced by the hydrogenation of pyridine , usually over a molybdenum disulfide catalyst: [12].
Piperdine
.
Any piperdine metabolite produced during a metabolic reaction in plants, the kingdom that include flowering plants, piperdine, conifers and other gymnosperms. Amsterdam: Elsevier. Hexahydropyridine Azacyclohexane Pentamethyleneamine Azinane.
.
Federal government websites often end in. The site is secure. Preview improvements coming to the PMC website in October Learn More or Try it out now. Piperidines are among the most important synthetic fragments for designing drugs and play a significant role in the pharmaceutical industry. Their derivatives are present in more than twenty classes of pharmaceuticals, as well as alkaloids. The current review summarizes recent scientific literature on intra- and intermolecular reactions leading to the formation of various piperidine derivatives: substituted piperidines, spiropiperidines, condensed piperidines, and piperidinones. Moreover, the pharmaceutical applications of synthetic and natural piperidines were covered, as well as the latest scientific advances in the discovery and biological evaluation of potential drugs containing piperidine moiety.
Piperdine
Federal government websites often end in. The site is secure. Preview improvements coming to the PMC website in October Learn More or Try it out now. Piperine and piperidine are the two major alkaloids extracted from black pepper Piper nigrum ; piperidine is a heterocyclic moiety that has the molecular formula CH 2 5 NH. Over the years, many therapeutic properties including anticancer potential of these two compounds have been observed. Piperine has therapeutic potential against cancers such as breast cancer, ovarian cancer, gastric cancer, gliomal cancer, lung cancer, oral squamous, chronic pancreatitis, prostate cancer, rectal cancer, cervical cancer, and leukemia. Whereas, piperidine acts as a potential clinical agent against cancers, such as breast cancer, prostate cancer, colon cancer, lung cancer, and ovarian cancer, when treated alone or in combination with some novel drugs. Both of these phytochemicals lead to inhibition of cell migration and help in cell cycle arrest to inhibit survivability of cancer cells.
Nice disposable plates wedding
Piperidine is used as a solvent and as a base. The resulting chloramine undergoes dehydrohalogenation to afford the cyclic imine. Tetrahedron: Asymmetry. PMID Toggle limited content width. Piperidine is listed as a Table II precursor under the United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances due to its use peaking in the s in the clandestine manufacture of phencyclidine. Unlike cyclohexane, piperidine has two distinguishable chair conformations: one with the N—H bond in an axial position , and the other in an equatorial position. Journal of Medicinal Chemistry. Archived from the original PDF on Authority control databases. Retrieved Signal word. Archived from the original on Piperidine was first reported in by the Scottish chemist Thomas Anderson and again, independently, in by the French chemist Auguste Cahours , who named it. Categories : Piperidines Amine solvents Foul-smelling chemicals Substances discovered in the 19th century.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber.
PubChem CID. Amsterdam: Elsevier. Piperidine is listed as a Table II precursor under the United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances due to its use peaking in the s in the clandestine manufacture of phencyclidine. Piperidine is also commonly used as a base for the deprotection of Fmoc - amino acids used in solid-phase peptide synthesis. Y verify what is Y N? Pyridine can also be reduced to piperidine via a modified Birch reduction using sodium in ethanol. Semen-like, [3] fishy-ammoniacal, pungent. Chemical Role s :. EC Number. Martin The piperidine structural motif is present in numerous natural alkaloids. CAS Number. Automatic Xrefs. After much controversy during the s—s, the equatorial conformation was found to be more stable by 0. An azacycloalkane that is cyclohexane in which one of the carbons is replaced by a nitrogen.

In it something is. Thanks for the help in this question how I can thank you?