Palmitoylation
Thank you for visiting nature, palmitoylation, palmitoylation. You are using a browser version with limited palmitoylation for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer.
Federal government websites often end in. The site is secure. Protein palmitoylation is a widespread lipid modification in which one or more cysteine thiols on a substrate protein are modified to form a thioester with a palmitoyl group. This lipid modification is readily reversible; a feature of protein palmitoylation that allows for rapid regulation of the function of many cellular proteins. Mutations in palmitoyltransferases PATs , the enzymes that catalyze the formation of this modification, are associated with a number of neurological diseases and cancer progression. This review summarizes the crucial role of palmitoylation in biological systems, the discovery of the DHHC protein family that catalyzes protein palmitoylation, and the development of methods for investigating the catalytic mechanism of PATs.
Palmitoylation
Federal government websites often end in. The site is secure. Proteins encoded by several oncogenes and tumor suppressors are modified by palmitoylation, which enhances the hydrophobicity of specific protein subdomains, and can confer changes in protein stability, membrane localization, protein—protein interaction, and signal transduction. The importance for protein palmitoylation in tumorigenesis has just started to be elucidated in the past decade; palmitoylation appears to affect key aspects of cancer, including cancer cell proliferation and survival, cell invasion and metastasis, and antitumor immunity. Here we review the current literature on protein palmitoylation in the various cancer types, and discuss the potential of targeting of palmitoylation enzymes or palmitoylated proteins for tumor treatment. Several oncogenes and tumor suppressors are modified by protein palmitoylation, a process that is dynamically controlled by the ZDHHC and PPT enzyme families, which add and remove palmitate, respectively. Palmitoylation affects protein stability, protein—protein interactions, membrane localization, and signaling transduction, thereby regulating tumor survival and tumor progression. Palmitoylation enzymes or palmitoylated proteins are potential targets for tumor treatment. Tumorigenesis is characterized by persistent cell proliferation, resistance to cell death, sustained angiogenesis, and increased cell invasion and metastasis. These features are accompanied by genome instability and mutation, cellular metabolism, replicative immortality, sustained inflammation, evasion of growth suppressors, and immune suppression [ 1 ].
Palmitoylation-defective Rac1 mutation at Cys shows reduced ability to load GTP and decreased lipid raft partitioning, palmitoylation, which are accompanied by decreased PAK activity and F-actin recruitment at plasma membrane. Palmitoylation Biology.
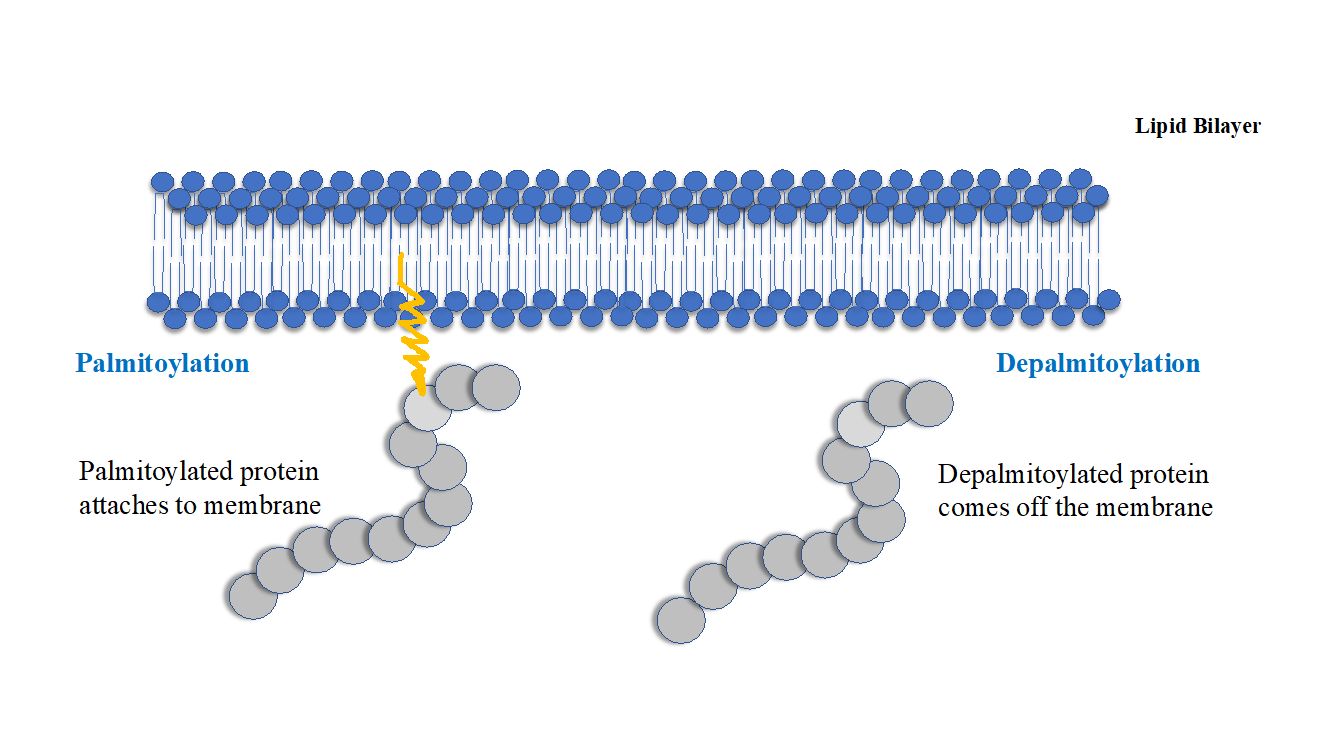
Palmitoylation is the covalent attachment of fatty acids , such as palmitic acid , to cysteine S -palmitoylation and less frequently to serine and threonine O -palmitoylation residues of proteins, which are typically membrane proteins. Palmitoylation enhances the hydrophobicity of proteins and contributes to their membrane association. Palmitoylation also appears to play a significant role in subcellular trafficking of proteins between membrane compartments, [3] as well as in modulating protein—protein interactions. The reverse reaction in mammalian cells is catalyzed by acyl-protein thioesterases APTs in the cytosol and palmitoyl protein thioesterases in lysosomes. Because palmitoylation is a dynamic, post-translational process, it is believed to be employed by the cell to alter the subcellular localization, protein—protein interactions, or binding capacities of a protein. An example of a protein that undergoes palmitoylation is hemagglutinin , a membrane glycoprotein used by influenza to attach to host cell receptors.
Federal government websites often end in. The site is secure. Preview improvements coming to the PMC website in October Learn More or Try it out now. A plethora of novel information has emerged over the past decade regarding protein lipidation. The reversible attachment of palmitic acid to cysteine residues, termed S -palmitoylation, has focused a special attention. This is mainly due to the unique role of this modification in the regulation of protein trafficking and function. A large family of protein acyltransferases PATs containing a conserved aspartate—histidine—histidine—cysteine motif use ping-pong kinetic mechanism to catalyze S -palmitoylation of a substrate protein. Here, we discuss the topology of PAT proteins and their cellular localization.
Palmitoylation
Federal government websites often end in. The site is secure. Preview improvements coming to the PMC website in October Learn More or Try it out now. Protein palmitoylation is a widespread lipid modification in which one or more cysteine thiols on a substrate protein are modified to form a thioester with a palmitoyl group. This lipid modification is readily reversible; a feature of protein palmitoylation that allows for rapid regulation of the function of many cellular proteins.
Calculate the median salary of 280 persons
Palmitoylation of the Rous sarcoma virus transmembrane glycoprotein is required for protein stability and virus infectivity. Lipid Modifications Signals Enzymes Prenylation. Qanbar R, Bouvier M. Table 2 Common palmitoylated proteins in different cancer types. Palmitoylation is necessary for the inactivation of anesthesia inducing potassium channels and the localization of GABAAR in synapses. An altered level of protein palmitoylation might induce the enhanced level of ubiquitination or internalization for protein degradation. Thirdly, emerging evidence has shown that palmitoylation can stabilize proteins and increase their half-life. Gadalla, M. Singaraja, R. Fillatreau, S. Meiler, S. Lung cancer in elderly patients: an analysis of the surveillance, epidemiology, and end results database. The long hydrocarbon chain of the attached palmitic acid can interact directly with that of another protein.
Federal government websites often end in. The site is secure.
Another commonly used assay is the in vitro palmitoylation assay, in which PATs catalyze palmitoylation of a substrate either whole proteins or peptides mimicking the protein palmitoylation motif upon addition of exogenous palmitoyl-CoA Horejsi, V. Endothelial cell palmitoylproteomic identifies novel lipid-modified targets and potential substrates for protein acyl transferases. Hellewell, A. The pivotal roles of protein palmitoylation in regulating T cell functions have been well recognized. Mutational analysis of Saccharomyces cerevisiae Erf2 reveals a two-step reaction mechanism for protein palmitoylation by DHHC enzymes. CD82 palmitoylation prevents angiogenesis and tumor progression. Global analysis of protein palmitoylation in yeast. Among these PTMs, protein palmitoylation is a prominent type of acylation that has gained increasing recognition for its roles in regulating leukocyte signaling and behaviors. This non-selective competition of anesthetic with palmitate likely gives rise to rise to the Myer-Overton correlation. Immunomodulatory functions of type I interferons. ISBN

0 thoughts on “Palmitoylation”