Lewis structure for ca
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms.
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties. Physical Properties. Intensive vs.
Lewis structure for ca
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A Lewis electron dot symbol or electron dot diagram or a Lewis diagram or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. It does not matter what order the positions are used. Figure 1. Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. Lewis symbols can also be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium: Likewise, they can be used to show the formation of anions from atoms, as shown below for chlorine and sulfur: Figure 2 demonstrates the use of Lewis symbols to show the transfer of electrons during the formation of ionic compounds. Figure 2. Cations are formed when atoms lose electrons, represented by fewer Lewis dots, whereas anions are formed by atoms gaining electrons. The total number of electrons does not change. The valence electron configuration for aluminum is 3 s 2 3 p 1. So it would have three dots around the symbol for aluminum, two of them paired to represent the 3 s electrons or three single dots around the atom :.
Draw the Lewis electron dot diagram for each element. So it would have three dots around the symbol for aluminum, two of them paired to represent the 3 s electrons or three single dots around the atom :.
.
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A Lewis electron dot symbol or electron dot diagram or a Lewis diagram or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. It does not matter what order the positions are used. Figure 1. Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table.
Lewis structure for ca
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A Lewis electron dot diagram or electron dot diagram, or a Lewis diagram, or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom.
Reveal antonym
What column of the periodic table has Lewis electron dot diagrams with two electrons? The next atom, lithium, has an electron configuration of 1 s 2 2 s 1 , so it has only one electron in its valence shell. Addition and Subtraction Operations. Naming Ionic Hydrates. Main Group Elements: Periodic Trends. Quantum Numbers: Number of Electrons. MO Theory: Bond Order. Guided course. Dipole Moment. Quantum Numbers: Principal Quantum Number. Periodic Trend: Metallic Character.
In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. In this section, we will explore the typical method for depicting valence shell electrons and chemical bonds, namely Lewis symbols and Lewis structures. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions.
Thermal Equilibrium. With nitrogen, which has three p electrons, we put a single dot on each of the three remaining sides:. Hydrogen Compounds. Cell Potential: Standard. Born Haber Cycle. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. Quantum Numbers: Spin Quantum Number. Bohr Equation. Factors Influencing Rates. Intro to Crystal Field Theory. Nuclear Chemistry 2h 34m. The third electron will go on another side of the symbol:. Figure 2. Intermolecular Forces and Physical Properties.

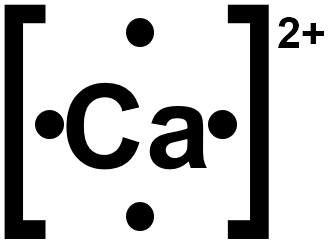
Bravo, this excellent phrase is necessary just by the way