Lewis structure for c2h4
Most stable structure is taken as the lewis structure of ethene. Hybridization of atoms in ethene molecue can be found from lewis structure. Each step of determining the lewis structure of ethene and hybridization are explained in this tutorial.
The chemical formula C 2 H 4 represents Ethylene. An alkene is a hydrocarbon with a Carbon-Carbon double bond. C 2 H 4 exists as a colorless gas and is flammable. Ethylene occurs naturally in petroleum natural gas. It inhibits growth in plants and promotes the ripening of fruits.
Lewis structure for c2h4
Hydrocarbons form an essential and inseparable portion of the science of chemistry. Be it petroleum, crude oil, or natural gas, the majority of hydrocarbons are found naturally in these fossil fuels. Apart from this, we can find them in synthetic polymers and other man-made plastic materials. They are organic in nature and as the name suggests, they are formed of only carbon and hydrogen. Sometimes, it also creates compounds with other varieties like sulfur, nitrogen, and so on. Although these are some of the simplest organic compounds we can come across, they have a varied range and differ in several physical and chemical properties. In this article, we will talk about one of the most common and widely used hydrocarbons: Ethylene C2H4. Well, C2H4 is a simple straight-chain hydrocarbon that bears a sweet aroma and has a colorless form. So, it is important for us to learn about C2H4 in detail to understand the nature of straight-chain hydrocarbons in a better manner. Carbon has a covalent nature when it comes to bonding with hydrogen and this leads to the formation of the different types of hydrocarbons that we see. From simplest ones like methane and benzene to some of the complex ones like natural rubber, we deal with several HCs in our daily lives. In organic chemistry, we find hydrocarbons of several types: straight-chain, cyclic, and even branched.
Temporary Hardness of Water. Look the figures to understand each step.
The bond angle between the orbitals is o with no free rotation about a carbon double bond. C2H4 is the chemical formula of a colourless and flammable gas known as Ethylene. It is said to be a hydrocarbon that has two carbon atoms connected to it with a double bond. It is lighter than air. Here, one 2p orbital does not change, and it will help form a pi bond. Molecular structure defines the arrangement of atoms in a molecule or ion. The bonded pair of hydrogen attached to carbon repels each other, and as a result, the figure thus formed is a trigonal planar.
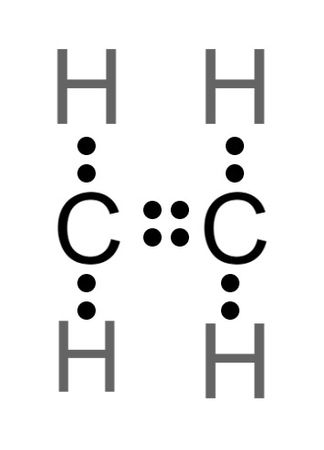
Ethane Lewis Dot Structure would refer to the structure formation of the compound ethane with chemical description. Detailed structure by explaining the facts shown by Lewis structure would be represented in this research. One ethane molecule consists of two carbon and six oxygen atoms. The chemical formula of the molecule is C2H6. The total number of valance electrons in this compound is It is very important to understand the calculation of valance electron ion making the Lewis dot structure if the molecules. The valance electrons are the main participants in formation of the binds between the different atoms. In the case of calculating the number of valance electrons of the each atom it is in- s needed to identify the number of valance electrons hold by the atoms. Therefore, let us find the total number of electrons in each of the carbon and hydrogen atoms. The total number of electrons in carbon is six and total number of election in one hydrogen atom is one.
Lewis structure for c2h4
In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. In this section, we will explore the typical method for depicting valence shell electrons and chemical bonds, namely Lewis symbols and Lewis structures. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons:. Electron dots are typically arranged in four pairs located on the four "sides" of the atomic symbol.
Cnn radyo dinle
Therefore, C 2 H 4 is a Planar. C2H4 Molecular Geometry Importance Molecular structure defines the arrangement of atoms in a molecule or ion. Below, That step are done. Hydrogen has only one electron in its valence shell. Law of Thermodynamics. Therefore, the final C 2 H 4 Lewis structure is given below. Chemical Bonding in Ethylene. Lewis structure helps to find out C2H4 molecular geometry as Lewis diagram determines the number of lone pairs and bond pairs a molecule comprises. The electron dot structure of ethene, C 2 H 4 :. Learn more topics related to Chemistry. An easy way to look at the Hybridization of Ethylene is to consider the electron domains on each Carbon atom.
Watch the video of Dr. Note that the C 2 H 4 Lewis dot structure involves sharing more than one pair of electrons. Video Transcript: OK, we're going to do one here, this is called ethene.
Temporary Hardness of Water. One carbon and two hydrogen atoms together form a triangular shape, and so do the other carbon and hydrogen atoms. Table of Content. So, the valence electrons being negatively charged have a tendency to repel each other within a molecule. Draw the electron dot structure of Propanone. Here, we can see that one carbon atom has its octet fulfilled the Octet rule has been discussed before. The least electronegative atom among the given molecule will be chosen as the central atom of the molecule or ion. There are no lone pairs on atoms in the lewis structure of ethene. Step 2: Now, that we have found out the total valence number, we get to check which atom is less electronegative. Leave a Reply Cancel reply Your email address will not be published.

Tell to me, please - where to me to learn more about it?
You have hit the mark. In it something is also I think, what is it good idea.