Lewis dot structure for becl2
BeCl2 lewis structure has a Beryllium atom Be at the center which is surrounded by two Chlorine atoms Cl. There are 2 single bonds between the Beryllium atom Be and each Chlorine atom Cl.
BeCl2 referred to as Beryllium Chloride, is an inorganic compound. It appears as white or yellow crystal solid at room temperature. It can exist in both monomeric and 1-D polymeric forms. The properties of beryllium chloride are similar to aluminum chloride owing to the diagonal relationship of beryllium with aluminum. The molar mass and melting point of beryllium chloride are The chemical bonding in Beryllium Chloride is studied by writing down its Lewis structure by following the Lewis approach. After lewis structure, there is a need of understanding its molecular geometry and hybridization of the central atom, Beryllium.
Lewis dot structure for becl2
.
Jay Rana.
.
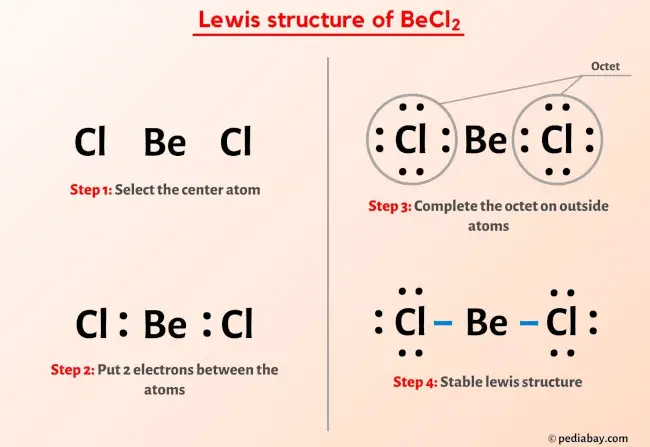
Ready to learn how to draw the lewis structure of BeCl2? Here, I have explained 6 simple steps to draw the lewis dot structure of BeCl2 along with images. The Beryllium atom Be is at the center and it is surrounded by 2 Chlorine atoms Cl. The Beryllium does not have lone pairs while both the Chlorine atoms have 3 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of BeCl2.
Lewis dot structure for becl2
BeCl2 referred to as Beryllium Chloride, is an inorganic compound. It appears as white or yellow crystal solid at room temperature. It can exist in both monomeric and 1-D polymeric forms. The properties of beryllium chloride are similar to aluminum chloride owing to the diagonal relationship of beryllium with aluminum.
On a roll sushi kawana
Beryllium will act as the central atom and chlorine atoms will surround it. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. Skip to content BeCl2 referred to as Beryllium Chloride, is an inorganic compound. These hybrid orbitals overlap with the atomic orbitals of surrounding atoms and hence, bond formation takes place. BeCl2 Lewis Structure. Bonding molecular orbitals are lower in energy and hence, more stable than individual atomic orbitals whereas antibonding molecular orbitals will be higher in energy than individual atomic orbitals and hence, less stable. We have started from its two-dimensional representation i. According to valence bond theory VBT , atomic orbitals of the central atom fuse together and form hybrid orbitals of equivalent energy. Now beryllium requires only 4 electrons to become stable. Therefore, the hybridization of the Beryllium atom in the Beryllium chloride is sp. The 2px and 2py atomic orbitals of the beryllium atom will combine with the 3px and 3py group orbital of a chlorine atom and gives two bonding and two antibonding molecular orbitals. Now, we have 16 valence electrons and we need to arrange these electrons in the Lewis structure of BeCl2. These sp hybrid orbitals of Beryllium atom will overlap with 3p orbitals of chlorine atoms and hence, sigma bond formation takes place between Beryllium and chlorine. According to this theory, atomic orbitals of similar energy and symmetry around the molecular axis combine to form molecular orbitals.
Drawing BeCl2 Lewis Structure is very easy.
The number of atomic orbitals combining will be equal to the number of molecular orbitals. This indicates that the beryllium Be and chlorine Cl are chemically bonded with each other in a BeCl2 molecule. For that, we have to study the Valence shell electron pair repulsion VSEPR theory to predict the shape of beryllium chloride. Now, we have 16 valence electrons and we need to arrange these electrons in the Lewis structure of BeCl2. According to valence bond theory VBT , atomic orbitals of the central atom fuse together and form hybrid orbitals of equivalent energy. Chlorine is group 17 element on the periodic table. In the above lewis dot structure of BeCl2, you can also represent each bonding electron pair : as a single bond. Now here the given molecule is BeCl2 beryllium dichloride and it contains beryllium atom Be and chlorine atoms Cl. The chlorine atom would like to have eight electrons around it to complete its octet. As chlorine is more electronegative than beryllium and hence, its energy will be relatively lowered than beryllium.

0 thoughts on “Lewis dot structure for becl2”