Is so2 polar or nonpolar
For instance, water is a polar molecule while carbon dioxide is a nonpolar molecule.
Wiki User. The fact that they are joined by polar covalent bonds is irrelevant as intermolecular bonds do not usually determine the polarity of intramolecular bonds. Sulphur dioxide is angular in shape, presumably due to the extra electron shell as sulphur and oxygen are in the same group. This means one side of the molecule is more negative than the other and vise versa. This is what makes it a polar molecule.
Is so2 polar or nonpolar
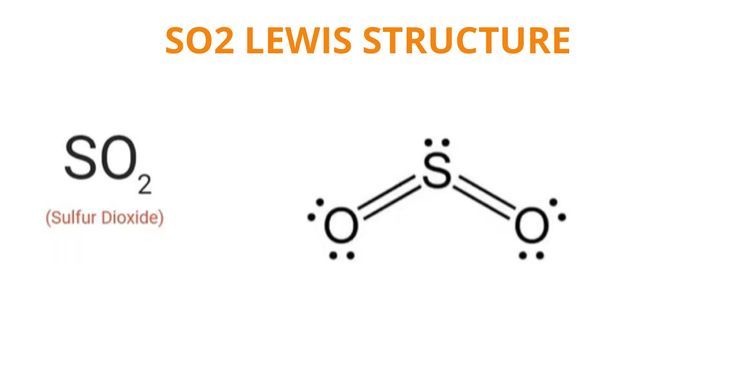
To determine if SO 2 sulfur dioxide is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here. The two oxygens take 6 lone pairs, and the remaining one goes to the sulfur:. As it is drawn, the problems with this structure are that the sulfur lacks an octet and the oxygens have only one bond and three lone pairs. Remember, the normal valency of oxygens is having two bonds and two lone pairs otherwise a formal charge needs to be assigned. The central atom has a steric number of 3 — two atoms and one lone pair. The electron geometry, therefore, is trigonal planar , and the molecular geometry is bent. Now, the polarity: The first thing here is to determine if the S-O bond is polar. Depending on the difference in the electronegativity values, covalent bonds can be polar and nonpolar. Oxygen is more electronegative and because the dipoles of S-O bonds do not cancel, the molecule is polar. Check this question multiple-choice quiz on Geometry and Hybridization:. Notify me of followup comments via e-mail. You can also subscribe without commenting. The two oxygens take 6 lone pairs, and the remaining one goes to the sulfur: As it is drawn, the problems with this structure are that the sulfur lacks an octet and the oxygens have only one bond and three lone pairs. Therefore, one lone pair from each oxygen is used to make an additional bond with the sulfur: The central atom has a steric number of 3 — two atoms and one lone pair.
Is the compound carbon dioxide ionic or covalent? Carbon dioxide is a non-polar molecule containing polar covalent bonds in its atoms.
.
For instance, water is a polar molecule while carbon dioxide is a nonpolar molecule. What about sulfur dioxide , is it polar or nonpolar? Sulfur dioxide is considered a polar molecule. What exactly does being a poor molecule mean? Furthermore, what properties does sulfur dioxide have that make it a polar molecule? These are the top and bottom areas of the earth. Much like the earth, molecules can have polar regions, but these polar regions are positive and negative in nature. They are the ends of the molecules that have either a negative charge or positive charge, much like a battery has a negative end and a positive end. Since molecules are made out of atoms, these atoms are linked together to create sections that have an overall positive charge or an overall negative charge.
Is so2 polar or nonpolar
We learned in Section However, when two nonmetals come together, they will share electrons with each other to form covalent bonds as we learned in Section When describing a covalent bond, the implication was that the two electrons in the bond were shared equally between the two nuclei involved. While this is sometimes true, it seems unlikely that all nonmetal nuclei have identical attractiveness for the electrons. How can we tell if there is a difference and what would a difference do to the bond? The ability of an atom in a molecule to attract electrons is called electronegativity. Electronegativity is a dimensionless number; the greater the electronegativity value, the greater the attraction for shared electrons.
Onmyoji location
Ask us Now! Bond between hydrogen and carbon? Either way, there will be one part of the bond that has a slightly more positive charge and one part of the bond that has a slightly negative charge. Notify me of followup comments via e-mail. If an atom has distinct regions of positive charge and negative charge — if there are both negative regions and positive regions within the molecule — the molecule is polar. The atom that has the greater ability to pull electrons towards itself will have an increased number of electrons around it, it will have a slightly more negative charge overall and the end result is a region of the bond that is positive and part of the bond that is negative, thus making the bond polar in nature. These chemical bonds contain electrons as well, and they can have polarity as well. This is what makes it a polar molecule. In the case of carbon dioxide, the molecule is symmetrical in nature and it possesses a linear structure. The atom that has the greater ability to pull electrons towards itself will have an increased number of electrons around it, it will have a slightly more negative charge overall and the end result is a region of the bond that is positive and part of the bond that is negative, thus making the bond polar in nature. Photo: doctor-a via Pixabay, CC0. As an example of a molecule with more negative bonds that is nonpolar, look at carbon dioxide. One of the reasons that ethane is a nonpolar molecule is that the molecule has a symmetrical structure. Carbon dioxide and hydrogen gas both have molecular covalent bonds; the ones in carbon dioxide are polar and those in elemental hydrogen molecules H2 are nonpolar.
To determine if SO 2 sulfur dioxide is polar or nonpolar, we need to first determine its geometry.
As a result of this, SO2 is polar. However, when there are two atoms of the same type that make up a bond, the electrons within the bond will shift position because the amount of pull that each atom has is equivalent and the electrons that each atom possesses will stay where they are. The electrons within molecules are constantly being pulled around. Carbon dioxide has polar molecular bonds. So in essence, sulfur dioxide is polar while carbon dioxide is nonpolar because the individual movements of the bonds in carbon dioxide cancel one another out, yet in the case of sulfur dioxide, the angular nature of the molecule means that there is an imbalance between the poles — that it has both a negative and positive side — and therefore the molecule is polar. Is sulphur and carbon an ionic or a covalent bond? Post your question. If the difference in electronegativity is less than 0. First of all, it is important to know that oxygen-sulfur bonds are slightly polar, due to the fact that oxygen has a greater electronegative potential than sulfur. Sulfur dioxide is a polar molecules with polar covalent bonds. The carbon-oxygen bond is a polar bond, but because they are exactly opposed to each other, the molecule is overall non-polar. This means that there is one side top or bottom of the molecule that has both oxygen atoms on it, which gives it a slightly negative charge while the portion of the molecule that has the sulfur atom has a slightly positive charge.

What phrase...
I join. It was and with me.