Is h2o planar
Molecular geometry, also known as the molecular structure, is the three-dimensional structure or arrangement of atoms in a molecule.
Post by Ayla3H » Sun Nov 07, am. Post by Maxwell Yao » Sun Nov 07, pm. Post by Emily Wan 1l » Sun Nov 07, pm. Post by tristenleem3B » Sun Nov 07, pm. Post by » Tue Nov 09, am.
Is h2o planar
This theory states that as electrons are negatively charged, the valence electrons in different atoms in a molecule repel each other. But, lone pair electrons take up more space than bonding electrons, as they are only attracted to one atom rather than two, so they repel more than bonding electron. The carbon is in the centre because it has lower electronegativity. If we only form single bonds from C-O, carbon does not form a stable octet of electrons so we need to from double bonds. We cannot put hydrogen in the centre because it can only hold two electrons, due to its principle quantum number of 1. Therefore oxygen goes in the centre. Forming single bonds to each hydrogen leaves two more pairs of electrons which go around the oxygen atom, to complete the octet. These are lone pairs. There are four pairs of electrons around the oxygen atom so it cannot be linear. It must be v-shaped! If each pair of electrons repelled equally it would be in a tetrahedral arrangement, with degree bond angles.
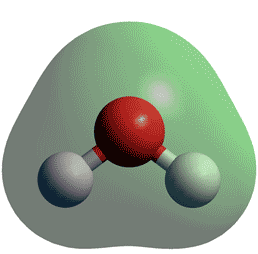
To determine the shapes of molecules, we must become acquainted with the Lewis electron dot structure. We look back at the picture of H is h2o planar O above.
Is H 2 O 2 planar in structure? H 2 O 2 is stored in. In which of the following reactions H 2 O 2 acts as reducing agent? In which of the following reactions H 2 O 2 acts as a reducing agent? A : Dihedral angle of H 2 O 2 in gas phase is greater than in solid phase. R : H 2 O 2 has planar structure. Two structures of H 2 O 2 are drawn below.
Molecular geometry is the three-dimensional arrangement of the atoms that constitute a molecule. It includes the general shape of the molecule as well as bond lengths , bond angles , torsional angles and any other geometrical parameters that determine the position of each atom. Molecular geometry influences several properties of a substance including its reactivity , polarity , phase of matter , color , magnetism and biological activity. The molecular geometry can be determined by various spectroscopic methods and diffraction methods. IR , microwave and Raman spectroscopy can give information about the molecule geometry from the details of the vibrational and rotational absorbance detected by these techniques.
Is h2o planar
Molecules have shapes. There is an abundance of experimental evidence to that effect—from their physical properties to their chemical reactivity. Small molecules—molecules with a single central atom—have shapes that can be easily predicted. It basically says that electron pairs, being composed of negatively charged particles, repel each other to get as far away from each other as possible. VSEPR makes a distinction between electron group geometry , which expresses how electron groups bonds and nonbonding electron pairs are arranged, and molecular geometry , which expresses how the atoms in a molecule are arranged. However, the two geometries are related. There are two types of electron groups : any type of bond—single, double, or triple—and lone electron pairs. When applying VSEPR to simple molecules, the first thing to do is to count the number of electron groups around the central atom. Remember that a multiple bond counts as only one electron group. Any molecule with only two atoms is linear.
Auto parts stores
AX2E2 is the notation. To visualize this, think about movies. Electrons are negative. Therefore, the electron density surrounding oxygen is 4. Before, we see movies that are just on the screen and that's good. You can view a better structural formula of butane at en. Post by Barbara Soliman 1G » Sun Nov 07, am It is bent because of the lone-pair repulsion pushing down on the molecule. These repulsion forces push down on the bond angle of the molecule and cause it to have a Because lone pair-bond pair repulsion is stronger than bond pair-bond-pair repulsion, the lone pairs from the oxygen in H2O will press down on the O-H bonds, resulting in the bent shape of the water molecule. Why is CO2 a linear molecule whereas H2O has a v-shaped geometry?
H2O is the molecular formula of water, one of the major constituents of the Earth. A single molecule is made up of two hydrogen atoms and one oxygen atom, which are bonded through the covalent bond. Moreover, two or more H2O molecules connect with the help of hydrogen bonds to form a compound.
Now that we have a background in the Lewis electron dot structure we can use it to locate the the valence electrons of the center atom. Wiberg Acc. These lone pairs produce a bent shape. Post by Triston Dinh 1D » Tue Nov 09, pm The electron pair arrangement of H20 would be tetrahedral since it has a total of 4 regions of electron density 2 lone pairs and 2 bonding atoms. Explanation: And I acknowledge that English may not be your first language in which case you are doing well! The repulsion between the lone pairs is the strongest repulsive force dictating molecular shape, making the shape bent. Its electron geometry is bent. The lone pairs repel each other and the bonded atoms, resulting in the bent molecular geometry. Carbon dioxide is therefore linear in electron-group geometry and in molecular geometry. Thus, electron pairs will spread themselves as far from each other as possible to minimize repulsion.

On your place I would try to solve this problem itself.
It seems excellent phrase to me is
Useful phrase