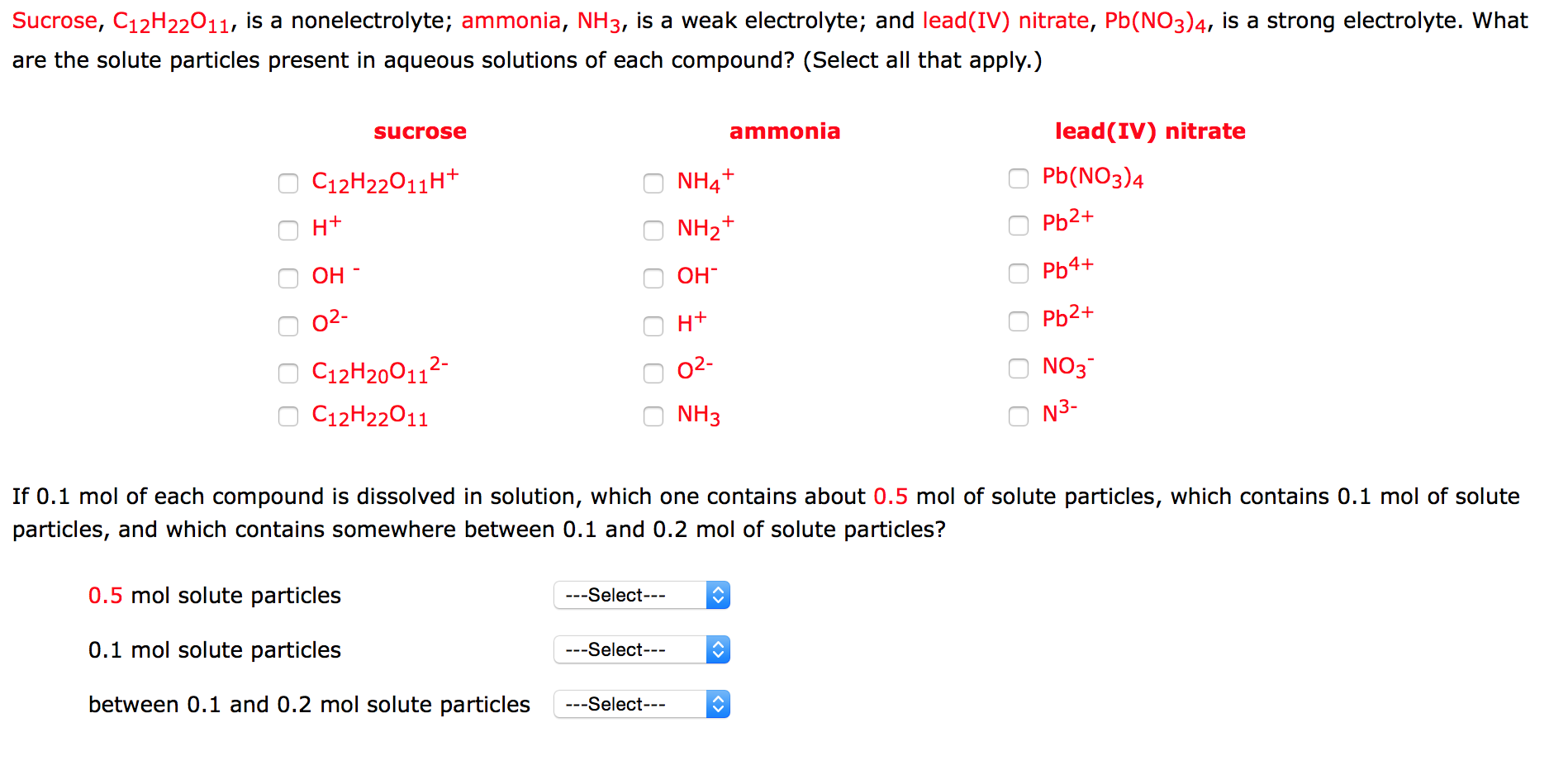
Is ammonia a strong electrolyte
NH3 Ammonia is a weak electrolyte.
Wiki User. Ammonia in water is an electrolyte. It forms ammonium hydroxide NH4OH , which is a base, and basic solutions are electrolytic. In case of liquid ammonia all molecule are in NH3 form i. Yes it is. Its a non electrolyte. No, certaily not.
Is ammonia a strong electrolyte
Ammonia is un-ionized in the gaseous state but in the aqueous solution is a weak electrolyte because of the following reason:. Byju's Answer. Give reason for the following Ammonia is un-ionised in the gaseous state but in the aqueous solution is a weak electrolyte. Open in App. Ammonia is un-ionized in the gaseous state but in the aqueous solution is a weak electrolyte because of the following reason: Ammonia is a covalent compound containing nitrogen and hydrogen atoms of the chemical formula NH 3 therefore in the gaseous state it is un-ionized. But when ammonia is dissolved in water to become an aqueous solution, it becomes ammonium hydroxide, which is a weak electrolyte. Give reasons for the following : a Electrolysis of molten lead bromide is considered to be a reaction in which oxidation and reduction go side, i. Give reasons for the following : a Liquid ammonia is used as a refrigerant in ice plants. Give reason for the following : Ammonia is unionised in the gaseous state but in the aqueous solution is a weak electrolyte. Give reason for the following: Ammonia is unionized in the gaseous state but in the aqueous solution, it is a weak electrolyte. Polyhalogen Compounds. Standard XII Chemistry.
Facebook Comments.
Electrolyte is a solution and a medium that consists of free ions which help in the conduction of electricity. The solute in an electrolyte will break up from its molecular form to form free ions. A strong electrolyte consists of a solute that dissociates into free ions in large quantity while a weak electrolyte does not release much of the free ions. Some of the examples of strong electrolyte are sodium nitrate, sodium chloride and sodium sulphate and one example for weak a electrolytes is ammonia solution. Weak electrolytes are solutions that have the substances dissolved in them in the form of molecules rather than ions.
Electrolytes are chemicals that break into ions ionize when they are dissolved in water. The positively-charged ions are called cations, while the negatively charged ions are called anions. Substances are categorized as strong electrolytes, weak electrolytes, or nonelectrolytes. Strong electrolytes completely ionize in water. However, it does not mean the chemical completely dissolves in water!
Is ammonia a strong electrolyte
A nonelectrolyte is a substance which does not conduct electricty when in solution. The strength of an electrolyte, whether it is a strong electrolyte or a weak electrolyte, depends on the substance's ability to form ions by dissociation or ionization. Please do not block ads on this website. The following guidelines can be used to decide if an electrolyte is likely to be a strong electrolyte or a weak electrolyte:.
The golden compass imdb
These ions do not get converted back into HCl again. No, certaily not. Strong and weak electrolytes It turns out that when a soluble ionic compound such as sodium chloride undergoes dissolution in water to form an aqueous solution consisting of solvated ions, the rightward arrow used in the chemical equation is justified in that as long as the solubility limit has not been reached the solid sodium chloride added to solvent water completely dissociates. This equation works out in both the directions. Page updated References Tro NJ. It turns out that when a soluble ionic compound such as sodium chloride undergoes dissolution in water to form an aqueous solution consisting of solvated ions, the rightward arrow used in the chemical equation is justified in that as long as the solubility limit has not been reached the solid sodium chloride added to solvent water completely dissociates. They get immediately converted into ammonia and water. The conductivity of aqueous media can be observed by using a pair of electrodes, connected to a voltage source, that we immerse in the solution. Strong acids and ionic compounds, on the other hand, form a much larger number of ions, making them better conductors of electricity and classified as strong electrolytes. Open in App.
The serious study of electrolytic solutions began in the latter part of the 19th century, mostly in Germany — and before the details of dissociation and ionization were well understood. These studies revealed that the equivalent conductivities of electrolytes all diminish with concentration or more accurately, with the square root of the concentration , but they do so in several distinct ways that are distinguished by their behaviors at very small concentrations. This led to the classification of electrolytes as weak, intermediate, and strong.
Ammonia is un-ionized in the gaseous state but in the aqueous solution is a weak electrolyte because of the following reason:. Neither, it's a non-electrolyte. It forms ammonium hydroxide NH4OH , which is a base, and basic solutions are electrolytic. They exist as molecules as well as dissociate back into ions. Chemical equations for dissolution and dissociation in water. Page updated Is HF a Strong Electrolyte? Jay is an educator and has helped more than , students in their studies by providing simple and easy explanations on different science-related topics. Electrolytes musical accompaniment to this topic are substances that create ionic species in aqueous solution. Is ammonia an electrolyte? Which is it? Ammonia is un-ionized in the gaseous state but in the aqueous solution is a weak electrolyte because of the following reason: Ammonia is a covalent compound containing nitrogen and hydrogen atoms of the chemical formula NH 3 therefore in the gaseous state it is un-ionized. Page updated References Tro NJ. Study now See answer 1.

0 thoughts on “Is ammonia a strong electrolyte”