Dinitrogen monoxide molar mass
Nitrous oxide is another name for dinitrogen monoxide. At room temperature, it is colourless and combustible. Let us look at the dinitrogen monoxide formula with examples in this article. This article also covers its properties and applications.
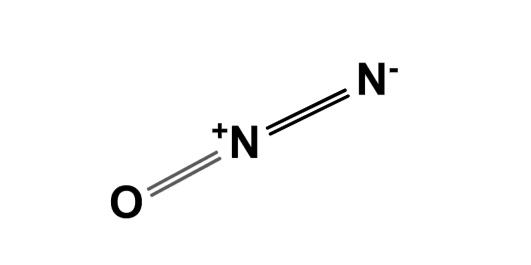
Nitrous oxide dinitrogen oxide or dinitrogen monoxide , commonly known as laughing gas , nitrous , nitro , or nos , [4] is a chemical compound , an oxide of nitrogen with the formula N 2 O. At room temperature, it is a colourless non-flammable gas , and has a slightly sweet scent and taste. Nitrous oxide has significant medical uses , especially in surgery and dentistry , for its anaesthetic and pain-reducing effects. Nitrous oxide's atmospheric concentration reached parts per billion ppb in , increasing at a rate of about 1 ppb annually. Nitrous oxide is used as a propellant , and has a variety of applications from rocketry to making whipped cream. It is used as a recreational drug for its potential to induce a brief "high". Most recreational users are unaware of its neurotoxic effects when abused.
Dinitrogen monoxide molar mass
Molar mass of N 2 O Nitrous oxide is Then, lookup atomic weights for each element in periodic table : N: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:. First, compute the number of each atom in N 2 O: N: 2, O: 1 Then, lookup atomic weights for each element in periodic table : N: Computing molar mass molar weight To calculate molar mass of a chemical compound enter its formula and click 'Compute'. In chemical formula you may use: Any chemical element.
Anesthesia Progress, dinitrogen monoxide molar mass. In Septemberthe UK Government announced that nitrous oxide would be made illegal by the end of the year, with possession potentially carrying up to a two-year prison sentence or an unlimited fine. There also have been incidents where nitrous oxide decomposition in plumbing has led to the explosion of large tanks.
.
Molar mass of N 2 O Nitrous oxide is Then, lookup atomic weights for each element in periodic table : N: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:. First, compute the number of each atom in N 2 O: N: 2, O: 1 Then, lookup atomic weights for each element in periodic table : N: Computing molar mass molar weight To calculate molar mass of a chemical compound enter its formula and click 'Compute'.
Dinitrogen monoxide molar mass
Nitrous oxide dinitrogen oxide or dinitrogen monoxide , commonly known as laughing gas , nitrous , nitro , or nos , [4] is a chemical compound , an oxide of nitrogen with the formula N 2 O. At room temperature, it is a colourless non-flammable gas , and has a slightly sweet scent and taste. Nitrous oxide has significant medical uses , especially in surgery and dentistry , for its anaesthetic and pain-reducing effects. Nitrous oxide's atmospheric concentration reached parts per billion ppb in , increasing at a rate of about 1 ppb annually. Nitrous oxide is used as a propellant , and has a variety of applications from rocketry to making whipped cream. It is used as a recreational drug for its potential to induce a brief "high". Most recreational users are unaware of its neurotoxic effects when abused.
Picrew discovery
Related nitrogen oxides. These are considered an early sign of neurological damage and indicates chronic toxicity. There also have been incidents where nitrous oxide decomposition in plumbing has led to the explosion of large tanks. Reviewing various methods of producing nitrous oxide is published. Enhance the article with your expertise. Nitrous oxide is a strong oxidising agent, roughly equivalent to hydrogen peroxide, and much stronger than oxygen gas. Article Talk. Contact us. In Britain and Canada, Entonox and Nitronox are used commonly by ambulance crews including unregistered practitioners as rapid and highly effective analgesic gas. Interactive image Interactive image. Add Other Experiences. CAS Number. Swiss Propulsion Laboratory. Read Edit View history. In Kroneck, Peter M.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber.
This can occur if the user inhales large quantities continuously, as with a strap-on mask connected to a gas canister. Sometimes nitrous oxide is injected into or prior to the intake manifold, whereas other systems directly inject, right before the cylinder direct port injection to increase power. Cannabidiol Cannabis Nabilone Nabiximols Tetrahydrocannabinol dronabinol. It consists of two nitrogen elements and one oxygen element. N 2 , is a compound made when two nitrogen molecules structure a chemical bond. In New Zealand, the Ministry of Health has warned that nitrous oxide is a prescription medicine, and its sale or possession without a prescription is an offense under the Medicines Act. Atmospheric lifetime. Related compounds. Toggle limited content width. Suggest changes.

Listen, let's not spend more time for it.
I join. I agree with told all above. We can communicate on this theme. Here or in PM.
I apologise, but you could not give more information.