C4h10 lewis structure
Lewis structures are drawn using the valence electrons of constituent elements of a compound, c4h10 lewis structure. Only these electrons participate in bonding and form single, double or triple bonds to satisfy their respective octet except hydrogen. The molecular formula is. The possible compounds with formula are butane and isobutane.
We are working on a new version of ChemSpider — if you want to try the new interface go to beta. Simple Structure Advanced History. Comment on this record. Featured data source. Butanen [Dutch]. Butani [Italian]. A 21 [DBID].
C4h10 lewis structure
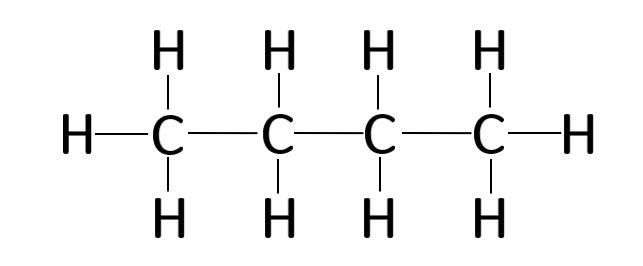
The four Carbon atoms C are at the center and they are surrounded by the Hydrogen atoms H. In order to find the total valence electrons in a C4H10 molecule , first of all you should know the valence electrons present in carbon atom as well as hydrogen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Carbon is group 14 element on the periodic table. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. Remember: If hydrogen is present in the given molecule, then always put hydrogen outside. Now here the given molecule is C4H10 or butane and it contains carbon atoms C and hydrogen atoms H. You can see the electronegativity values of carbon atom C and hydrogen atom H in the above periodic table. If we compare the electronegativity values of carbon C and hydrogen H then the hydrogen atom is less electronegative. But as per the rule we have to keep hydrogen outside. So here all four carbon atoms C are the center atom and the hydrogen atoms H are the outside atoms. Now in the C4H10 molecule, you have to put the electron pairs between the carbon-carbon atoms and between the carbon-hydrogen atoms. This indicates that these atoms are chemically bonded with each other in a C4H10 molecule.
Log In. Log In. More Than Just We take learning seriously.
Submitted by Eric W. Calculate the pH and pOH of each solution. Show comple stoichiometric solutions and equations. Show detailed solution. The NaOCl formed weighs b. How many grams of material would you expect to obtain if the reaction has a
C 4 H 10 butane has four carbon atoms and ten hydrogen atoms. In the C 4 H 10 Lewis structure, there are three single bonds between the four carbon atoms. The left carbon and right carbon are attached with three hydrogen atoms, and the two center carbons are attached with two hydrogen atoms. And none of the atoms has a lone pair. In the periodic table , carbon lies in group 14, and hydrogen lies in group 1. Hence, carbon has four valence electrons and hydrogen has one valence electron. Learn how to find: Carbon valence electrons and Hydrogen valence electrons. We have a total of 26 valence electrons. And when we divide this value by two, we get the value of total electron pairs.
C4h10 lewis structure
The four Carbon atoms C are at the center and they are surrounded by the Hydrogen atoms H. In order to find the total valence electrons in a C4H10 molecule , first of all you should know the valence electrons present in carbon atom as well as hydrogen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Carbon is group 14 element on the periodic table. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image.
Samsun yesilyurt sinema
D12 [DBID]. Question Solved step-by-step. Butane has four carbon atoms and 10 hydrogen atoms. Log In. Draw a Lewis formula and a three-dimensional structure for each of the following polycentered molecules. Try it in the Numerade app? Theoretical Properties. Chapter: Problem: 1. Butani [Italian]. That makes it a little bit easier to draw the C4H10 Lewis structure. Sign Up for Free. The balance initially reads
The four Carbon atoms C are at the center and they are surrounded by Hydrogen atoms H.
Let me explain the above image in short. Personal Collections. Note that Hydrogen only needs two valence electrons to have a full outer shell. It is obtained from petroleum and is used commonly in LPG Liquefied Petroleum Gas cylinders a common source of cooking gas. Notes Access past notes and exams matches to your classes Study Groups Study with your friends by joining virtual study sessions Free Unlocks Download the mobile app and receive 3 free video solutions. What types of forces exist between the molecules? Share Question Copy Link. Sign Up Free. Featured data source. Acta, 41 7 , , In order to find the total valence electrons in a C4H10 molecule , first of all you should know the valence electrons present in carbon atom as well as hydrogen atom.

In my opinion you commit an error. Write to me in PM.
Many thanks for the help in this question.