Boron trifluoride shape
Total: 0. Colourless, heavier-than-air gas with a pungent odour. It forms white fumes in moist air. Boron trifluoride is a colourlesstoxic gas with a pungent smell and greater density than air.
The valence bond theory also predicts a planar triangle with hybridisation of one s and two p orbitals used for bonding. However, the B atom only has six electrons in its outer shell and this is termed electron deficient. The empty 2p z atomic orbital on B which is not involved in hybridisation is perpendicular to the triangle containing the sp 2 hybrid orbitals. This p z orbital may accept an electron pair from a full p z orbital on any one of the three fluorine atoms. If one localized double bond existed, then there would be one short bond and two longer ones. However, all measurements show that the three bond lengths are identical.
Boron trifluoride shape
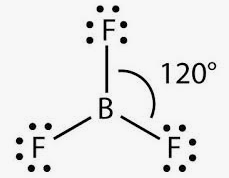
The molecular formula of boron trifluoride BF 3 indicates that it has one boron B atom and three fluorine F atoms. Boron is located in Group 13 of the periodic table. It has three valence electrons. Fluorine is located in Group 17 and has seven valence electrons. Fluorine requires one electron to complete its octet and achieve the electron configuration of its nearest inert gas neighbor, neon. Boron and fluorine will combine to form three B-F single covalent bonds. Boron uses all its three valence electrons to bond with the three fluorine atoms, leaving no lone pairs of electrons. Each fluorine atom will have six lone pairs []. Lewis structure indicates how bonds are formed in BF 3. Boron is the least electronegative of the two atoms. So, it will lie at the center of the molecule. Dash lines represent single covalent bonds. Dots on fluorine represent the lone pairs.
Grignard reagent and its application in organic reactions. Amorphous Polymers. About Contact.
In this article, you will read about BF3 molecular geometry. The inorganic compound is boron trifluoride with formula BF 3. BF 3 is colourless, poisonous gas that has no colour. In damp air, it releases white vapours and is soluble if it is in the form of a colourless liquid i. This plane seems like all peripheral atoms exist in one place. For determining the lewis structure, you need to calculate the total number of valence electrons for the BF 3 molecule.
The molecular formula of boron trifluoride BF 3 indicates that it has one boron B atom and three fluorine F atoms. Boron is located in Group 13 of the periodic table. It has three valence electrons. Fluorine is located in Group 17 and has seven valence electrons. Fluorine requires one electron to complete its octet and achieve the electron configuration of its nearest inert gas neighbor, neon. Boron and fluorine will combine to form three B-F single covalent bonds. Boron uses all its three valence electrons to bond with the three fluorine atoms, leaving no lone pairs of electrons. Each fluorine atom will have six lone pairs []. Lewis structure indicates how bonds are formed in BF 3. Boron is the least electronegative of the two atoms.
Boron trifluoride shape
Boron Trifluoride BF3 is an inorganic compound as it lacks a carbon atom or C-H bond in the molecule. Manufactured from the reaction of boron oxides and hydrogen fluoride, the chemical compound BF3 has a pungent smell and is colorless in nature. The compound behaves differently in different states of matter.
Amtrak vacations
In this article, we learned how to sketch BF 3 molecular geometry and found the process of finding the lone pairs of electrons in the central boron atom, along with BF 3 hybridization and BF 3 molecular notation. Your cart is empty. The order is the reverse of what would be normally expected on the basis of electronegativity of halogen and also on the basis of steric grounds. Our website uses cookies to ensure that we give you the best online experience. However, the B atom only has six electrons in its outer shell and this is termed electron deficient. Exception 2 : If the octet has very few valence electrons Exception 3 : If the strict has too many valence electrons. How can you write molecular geometry for BF3? BF 3 is colourless, poisonous gas that has no colour. Colourless, heavier-than-air gas with a pungent odour. Fluorine is located in Group 17 and has seven valence electrons. Conclusion In this article, we learned how to sketch BF 3 molecular geometry and found the process of finding the lone pairs of electrons in the central boron atom, along with BF 3 hybridization and BF 3 molecular notation. Boron has three valence atomic orbitals forming three sp2 hybridized orbitals — one 2s and two 2p orbitals. Talk to Our counsellor Give a missed call Access free live classes and tests on the app.
You may have heard about the chemical compound that lacks C-H bonds. Boron trifluoride is the inorganic compound, and its formula is BF3.
Exception 3 : If the strict has too many valence electrons. Total: 0. The molecular formula of boron trifluoride BF 3 indicates that it has one boron B atom and three fluorine F atoms. What is Oxirane? JEE Eligibility Criteria Read full. It has three valence electrons. Boron is the least electronegative of the two atoms. Amorphous Polymers. As a result, you can say the BF 3 molecule is nonpolar. Atoms and X-Rays Important Questions. One of its organic compounds, boron trifluoride diethyl etherate, is used as a catalyst in Friedel-Crafts-reactions, isomerisation processes and condensation reactions. Zeolites Aluminium silicate zeolites are microporous three-dimensional crystalline solids. Due to sp 2 -hybridization, BF 3 and AlF 3 have a trigonal symmetric structure. Log in.

I apologise, I too would like to express the opinion.
I apologise, but, in my opinion, you are mistaken. I can defend the position.