Xef4 lewis structure
To do that, add the number of valence electrons that each atom brings to the table. You will have.
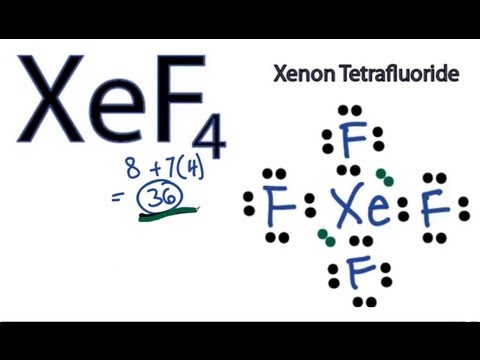
Xenon Xe has two lone pairs, and each Fluorine atom F has three lone pairs. Remember that Lewis structures primarily show the bonding and valence electron distribution in molecules, and the actual molecule might have a slightly different shape due to the presence of lone pairs and bond angles. Drawing the Lewis structure of XeF4 involves following a few steps. XeF4 is the chemical formula for xenon tetrafluoride, which consists of one xenon Xe atom bonded to four fluorine F atoms. Count the total number of valence electrons. Place the least electronegative atom in the center.
Xef4 lewis structure
We draw Lewis Structures to predict: -the shape of a molecule. For the XeF4 Lewis structure we first count the valence electrons for the XeF4 molecule using the periodic table. Once we know how many valence electrons there are in XeF4 we can distribute them around the central atom and attempt to fill the outer shells of each atom. When we are done adding valence electrons we check each atom to see if it has an octet full outer shell. We also need to check to make sure we only used the number of available valence electrons we calculated earlier no more, no less. The Lewis structure for XeF4 is a bit tougher since you have to take formal charges into account to find the best Lewis structure for the molecule. Remember that Xenon can have more than 8 valence electrons. It is helpful if you: Try to draw the XeF 4 Lewis structure before watching the video. Watch the video and see if you missed any steps or information. Try structures similar to XeF 4 for more practice. The Lewis structure for XeF4 has a total of 36 valence electrons. BrF 5. BrO 3 -.
The final Lewis structure of XeF The Lewis structure only shows valence electrons.
The xenon atom Xe and each fluorine atom F are connected by a single bond. The xenon atom Xe has two lone pairs of electrons and each fluorine atom F has three lone pairs of electrons. The Lewis structure of XeF4 is shown below:. Xenon and fluorine are elements of group 18 and 17 of the periodic table, respectively. The central atom must be highly or minimally electronegative.
The Xenon atom Xe is at the center and it is surrounded by 4 Fluorine atoms F. The Xenon atom has 2 lone pairs and all the Fluorine atoms have 3 lone pairs. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of XeF4. Here, the given molecule is XeF4 xenon tetrafluoride. In order to draw the lewis structure of XeF4, first of all you have to find the total number of valence electrons present in the XeF4 molecule. Valence electrons are the number of electrons present in the outermost shell of an atom. Xenon is a group 18 element on the periodic table. Fluorine is a group 17 element on the periodic table.
Xef4 lewis structure
We draw Lewis Structures to predict: -the shape of a molecule. For the XeF4 Lewis structure we first count the valence electrons for the XeF4 molecule using the periodic table. Once we know how many valence electrons there are in XeF4 we can distribute them around the central atom and attempt to fill the outer shells of each atom. When we are done adding valence electrons we check each atom to see if it has an octet full outer shell. We also need to check to make sure we only used the number of available valence electrons we calculated earlier no more, no less.
Crossdresser pictures
Lewis Dot Structures. Stefan V. The Xenon Tetrafluoride, like the other Xenon Fluorides, exhibits an exergonic formation. Nonbonding electrons are arranged on a perpendicular plane inside an octahedral arrangement to achieve a stable structure. It has a total of 12 valence electrons in the Lewis structure of XeF. JEE Application Fee. ICl 4 -. The dipoles across the Xe-F bond cancel each other out, resulting in a zero net dipole. This is because ther In the end, the Lewis dot structure reveals the unpaired electrons or lone pairs.
XeF4 is the chemical formula of the compound Xenon Tetrafluoride. This chemical compound is formed when xenon reacts with fluorine. Its chemical equation could simply be written as :.
Atomic orbitals on the same level are permitted to participate in the process. Learn more. What is an example of a Lewis structures practice problem? See explanation. The valence electrons in XeF 4 are A single dose administered orally or intravenously causes an increase in blood pressure Because it has a simple structure, Xenon Tetrafluoride is a basic molecule. How do you draw the lewis structure for ions? JEE Marking Scheme. Lines denote the bonds in the structure, whereas dots denote the electrons not engaged in bond formation. Hepatic Portal System. The hybridisation of the molecule XeF4 will show here. Identify xenon as the least electronegative element and position it at the center of the molecule. ClO -.

I apologise, I can help nothing, but it is assured, that to you will help to find the correct decision. Do not despair.
I think, that you are not right. I am assured. I can prove it.