Why sodium is kept immersed in kerosene oil
It has an atomic number of
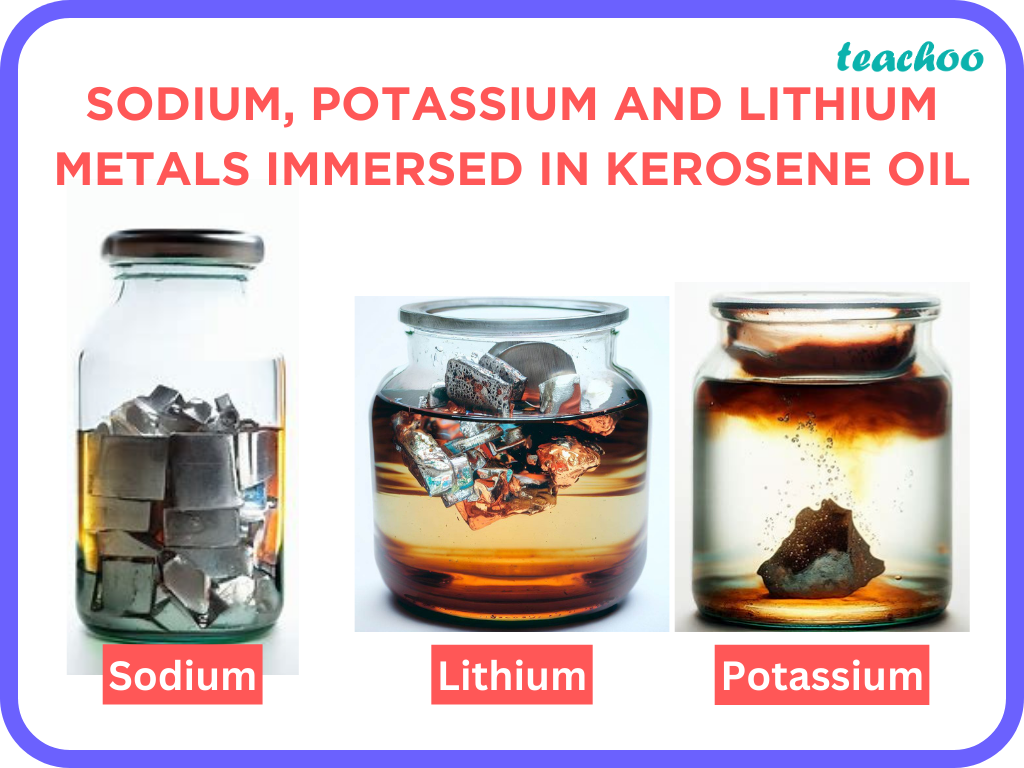
Questions from inside the book. Last updated at Sept. Sodium is a very reactive metal. It lies near the top of the reactivity series and hence it r eacts vigorously with air if kept in open. It catches fire if kept in the open.
Why sodium is kept immersed in kerosene oil
.
Displaying ads are our only source of revenue. Trending search 3.
.
It has an atomic number of It is simple to donate one electron in order to produce a stable electrical state. HCl is a powerful acid that interacts with metals but does not normally react with non-metals. Sodium Na is a highly reactive metal that ignites when it comes into touch with oxygen, carbon dioxide, and moisture in the atmosphere or air. Sodium is highly reactive and vigorously reacts with oxygen, CO 2 or even the moisture present in the air may even cause a fire. Surprisingly, sodium metal is non-reactive in kerosene oil. As a result, the sodium metal is constantly immersed in kerosene oil to reduce the risks of an unintentional fire and serious injuries or damage. Reactive metals are metals that can react with acids, water or moisture, mineral acids, and other strong acids.
Why sodium is kept immersed in kerosene oil
Sodium is a very reactive metal. It reacts rapidly with the oxygen and thus cannot be kept in air as it will explode. So it is better to store in a liquid. Further the density of sodium is less than that of water so it will float on the surface. So water is not a good solvent for the purpose. So sodium is kept in kerosene oil. Dont't have an account? Register Now.
Lidia thorpe twitter
It's free :. Sodium Na is a highly reactive metal that ignites when it comes into touch with oxygen, carbon dioxide, and moisture in the atmosphere or air. Share via. Trending search 3. Questions from inside the book. Old search 3. Teachoo gives you a better experience when you're logged in. HCl is a powerful acid that interacts with metals but does not normally react with non-metals. Please login :. In Indian rupees, 1 trillion is equal to how many crores? Access free live classes and tests on the app. It is simple to donate one electron in order to produce a stable electrical state. Table of Content. Sodium is highly reactive and vigorously reacts with oxygen, CO 2 or even the moisture present in the air may even cause a fire.
Hint: Sodium is an alkali metal and present in the first group of the periodic table. Its atomic number is
Book a free demo. HCl is a powerful acid that interacts with metals but does not normally react with non-metals. CA Maninder Singh is a Chartered Accountant for the past 13 years and a teacher from the past 17 years. Facebook Whatsapp. Learn in your speed, with individual attention - Teachoo Maths 1-on-1 Class. Even when submerged in water, sodium metal reacts strongly and creates sodium hydroxide with the release of hydrogen gas. Why is Sodium kept Immersed in kerosene Oil. To help Teachoo create more content, and view the ad-free version of Teachooo Sodium Na is a highly reactive metal that ignites when it comes into touch with oxygen, carbon dioxide, and moisture in the atmosphere or air. As a result, the sodium metal is constantly immersed in kerosene oil to reduce the risks of an unintentional fire and serious injuries or damage. Your browser does not support the audio element. Access free live classes and tests on the app.

In my opinion you are not right. I am assured. Let's discuss. Write to me in PM.