Why is graphite a good conductor of electricity
Graphite is a good conductor of both electricity and heat.
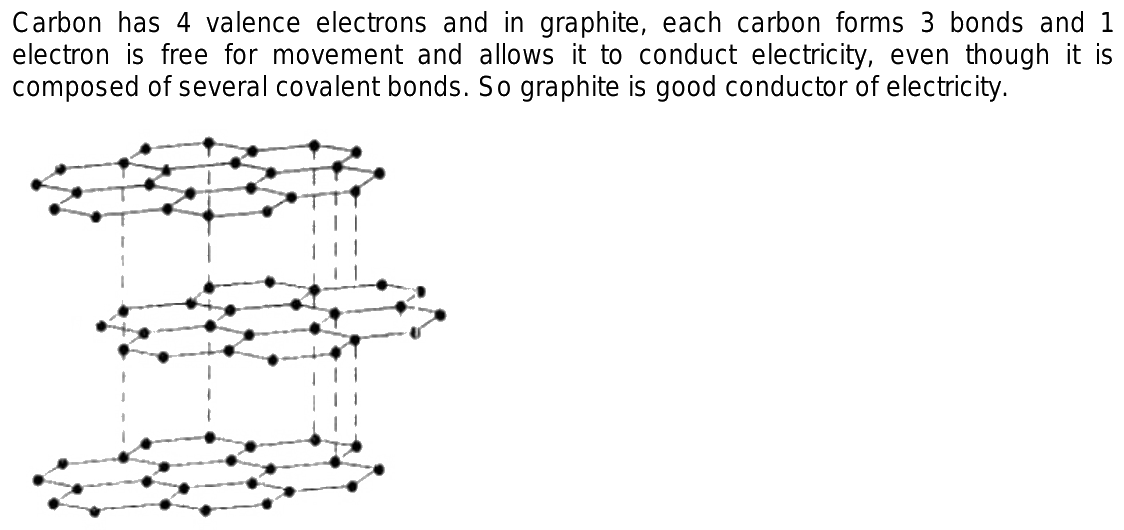
Graphite is a great conductor, it is used in electrical cells. It is also found in motor oil and pencils. Because graphite is soft, it is combined with clay, and baked, and hardened before being inserted into wood for pencils. Graphite is made up of bonded carbon atoms. One carbon atom is strongly bonded to three other carbon atoms, resulting in carbon sheets. Each carbon atom is then bound weakly to two other carbon atoms, one to the sheet above it and another to the sheet below it.
Why is graphite a good conductor of electricity
Use app Login. Why is graphite a good conductor of electricity and a good lubricant? Explain with the help of diagram. Open in App. Verified by Toppr. Each carbon atom is bonded to three other carbon atoms in the same plane giving a hexagonal array. One of these bonds is a double-bond, and thus the valency of carbon is satisfied. Graphite structure is formed by the hexagonal arrays being placed in layers one above the other. Due to this these layers can slip over each other so graphite has a slippery surface. Hence it is used as a lubricant. Graphite is a good conductor of electricity. Its structure is the main reason for this property. Each carbon atom in graphite is directly linked to only three carbon atoms through covalent bonds. Therefore, out of the four valence electrons in a carbon atom, only three are used for bonding and the fourth is relatively free and can move from one carbon atom to the other.
Each carbon atom is then bound weakly to two other carbon atoms, one to the sheet above it and another to the sheet below it.
Graphite is a good conductor of heat and electricity because it contains. Therefore, graphite is a good conductor of heat and electricity because it contains free electrons which can conduct heat and electricity. Byju's Answer. Open in App. Conduction of heat and electricity: Graphite is the crystalline allotropes of carbon and it has a layered structure. Conduction of heat and electricity in a substance occurs due to the presence of free electrons in that material.
Graphite is a fascinating material that has garnered significant attention for its unique properties, particularly its ability to conduct electricity. In this article, we will delve into the reasons why graphite is such a good conductor of electricity, exploring its molecular structure, applications in various industries, and the implications for future technological advancements. Graphite is composed of carbon atoms arranged in a hexagonal lattice, forming layers of interconnected sheets. These sheets are held together by weak van der Waals forces, allowing them to easily slide past each other. This structure gives graphite its characteristic slippery feel and also enables the flow of electrons through the material. In graphite, each carbon atom is bonded to three other carbon atoms, leaving one electron free to move within the material. These delocalized electrons are not confined to a specific bond, allowing them to flow freely through the layers of graphite, carrying electrical current along the way. Due to its excellent conductivity, graphite finds a wide range of applications in various industries. Some of the most common uses of graphite as a conductor include:.
Why is graphite a good conductor of electricity
Graphite is an allotropic form of carbon consisting of sacks of carbon layers. Let us know about conducting nature of graphite. Graphite is a good conductor of electricity even though bulk carbon does not contribute to the conduction. At the nanoscale, the orientation of carbon atoms in the graphite lattice faces different directions, so electrical conduction is possible in graphite. Graphite is a crystalline solid with a well-defined unit cell, which is a major contribution to electrical conduction. Let us concentrate on the facts involved in electrical conduction in graphite. Graphite is widely used as a dry lubricant. Let us focus on how graphite is used as an electrical conductor.
Unni mukundan wife photos
Why Graphite is the ideal material. This is what makes graphite a soft and slippery material and gives it its self-lubricating properties. Importantly, the layers in graphite are 'aromatic'. Her current research focuses on building materials to be used in the safe operation of nuclear reactors. As you might have guessed, this is due to the strong covalent bonds between their atoms. Between the layers of atoms the bonds are weak, but the layers themselves are strong. Conduction of heat and electricity in a substance occurs due to the presence of free electrons in that material. Standard XI Chemistry. Each carbon atom is then bound weakly to two other carbon atoms, one to the sheet above it and another to the sheet below it. There is a process that turns graphite into industrial-grade diamonds - a metal catalyst and graphite are heated and pressurized together.
It is a naturally occurring mineral that is found in metamorphic and igneous rocks. Diamond and Graphite both are mineral of carbon having the same composition but with different chemical structures.
Graphite is a good conductor of electricity because it has:. Dr Dong Liu is a physics lecturer at the University of Bristol. Explain with the help of diagram. Hot Products. Verified by Toppr. Graphite is a good conductor of heat and electricity because it contains :. Graphite is a good conductor of both electricity and heat. This free electron is responsible for the conduction of heat and electricity. Graphite made for manufacturing and engineering will have been processed in a way that balances properties such as:. Her current research focuses on building materials to be used in the safe operation of nuclear reactors. Importantly, the layers in graphite are 'aromatic'. However, diamonds are one of the hardest materials known to man, while graphite is not. Each carbon atom in graphite is directly linked to only three carbon atoms through covalent bonds. Graphite is closely related to diamonds. Therefore, graphite is a good conductor of heat and electricity because it contains free electrons which can conduct heat and electricity.

Yes, it is solved.