Which of the following is an example of homogeneous mixture
Key Points. Last updated on Nov 22, Get Started.
The correct answer is Salt solution. Key Points. Additional Information Homogeneous mixture. Last updated on Nov 8, Bihar police Constable Notification will be released soon.
Which of the following is an example of homogeneous mixture
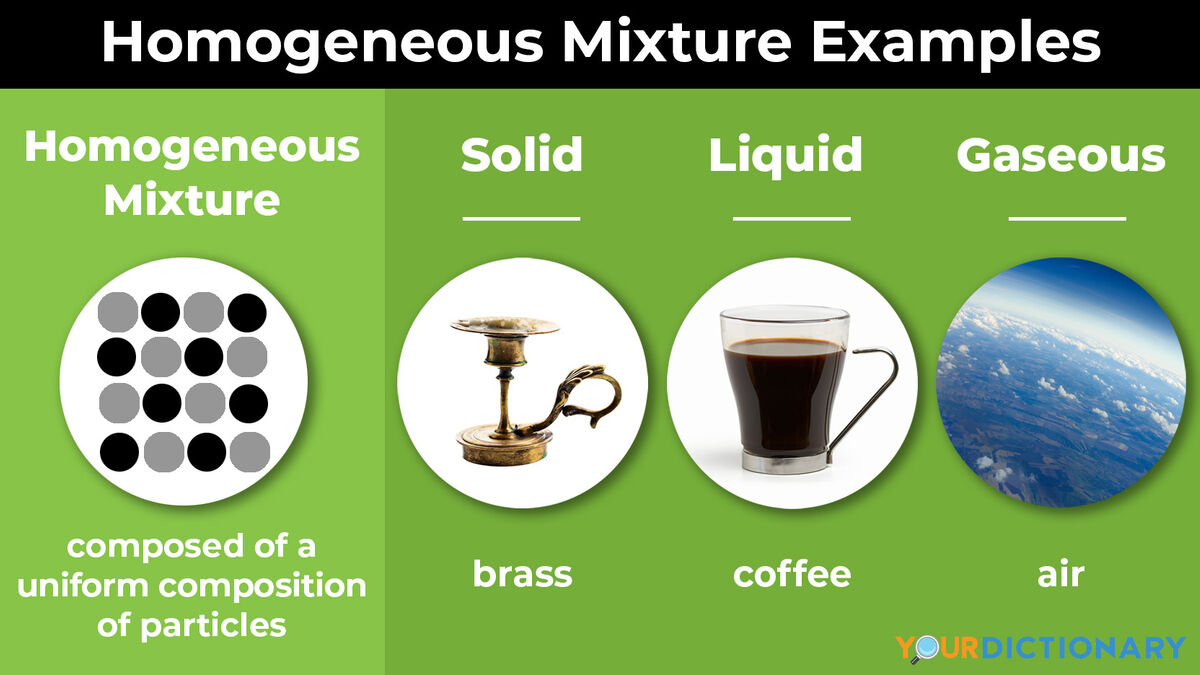
When you combine two or more materials, you form a mixture. In chemistry, a mixture is a combination that does not produce a chemical reaction. There are two categories of mixtures: homogeneous mixtures and heterogeneous mixtures. Here's a closer look at these types of mixtures and examples of mixtures. Homogeneous mixtures appear uniform to the eye. They consist of a single phase, be it liquid, gas, or solid, no matter where you sample them or how closely you examine them. The chemical composition is the same for any sample of the mixture. Heterogeneous mixtures are not uniform. If you take two samples from different parts of the mixture, they will not have an identical composition. You can use a mechanical method to separate components of a heterogeneous mixture e. Sometimes these mixtures are obvious, where you can see different types of materials in a sample. For example, if you have a salad, you can see different sizes and shapes and types of vegetables. In other cases, you need to look more closely to recognize this mixture.
Delhi Higher Judicial Service. The disease caused by breathing polluted air is:. Bihar Police Forest Guard.
.
The major component of a solution is called the solvent. The minor component of a solution is called the solute. By major and minor we mean whichever component has the greater or lesser presence by mass or by moles. Sometimes this becomes confusing, especially with substances with very different molar masses. However, here we will confine the discussion to solutions for which the major component and the minor component are obvious. Solutions exist for every possible phase of the solute and the solvent. In all cases, however, the overall phase of the solution is the same phase as the solvent. A solution is made by dissolving 1.
Which of the following is an example of homogeneous mixture
The terms heterogeneous and homogeneous refer to mixtures of materials in chemistry. The difference between heterogeneous and homogeneous mixtures is the degree to which the materials are mixed together and the uniformity of their composition. A homogeneous mixture is a mixture in which the components that make up the mixture are uniformly distributed throughout the mixture. The composition of the mixture is the same throughout. There is only one phase of matter observed in a homogeneous mixture at a time. So, you wouldn't observe both a liquid and a gas or a liquid and a solid in a homogeneous mixture.
Express vpn full apk cracked
Heterogeneous mixtures are not uniform. Telangana High Court Record Assistant. BSF RO. If one mole of carbon atoms weighs 12 gram, what is the mass in gram of 1 atom of carbon? More Chemistry Questions Q1. JSSC Stenographer. Rajasthan SET. More General Science Questions Q1. Kolkata Police Constable. Rajasthan Gram Vikas Adhikari. BSF SI. IB Security Assistant. Chandigarh JBT. Examples of homogeneous mixtures are rainwater, vinegar, air, saline solution, etc.
Many people enjoy a cup of coffee at some point during the day. Some may drink it black, while others may put cream or some dairy substitute and sugar in their coffee. High-end coffee drinks can be purchased at espresso stands either sit-down or drive-through.
English Hindi. Punjab Patwari. NWDA Exam. Maharashtra Nagar Parishad Fire Officer. Important Exam. Jharkhand High Court Assistant. Eugenics is the study of:. MH SET. RPF SI. Maharashtra Nagar Parishad Engineering Services.

It agree, very much the helpful information
I am assured, what is it � a lie.