What is the specific heat of a substance
If a swimming pool and wading pool, both full of water at the same temperature, were subjected to the same input of heat energy, the wading pool would certainly rise in temperature more quickly than the swimming pool. The heat capacity of an object depends both on its mass and its chemical composition.
Heat capacity is an extensive property, so it scales with the size of the system. For example, if it takes 1, J to heat a block of iron, it would take 2, J to heat a second block of iron with twice the mass as the first. The heat capacity of most systems is not a constant. Rather, it depends on the state variables of the thermodynamic system under study. In particular, it is dependent on temperature itself, as well as on the pressure and the volume of the system, and the ways in which pressures and volumes have been allowed to change while the system has passed from one temperature to another. The temperature dependence is why the definition a calorie is formally the energy needed to heat 1 g of water from Different measurements of heat capacity can therefore be performed, most commonly at constant pressure and constant volume.
What is the specific heat of a substance
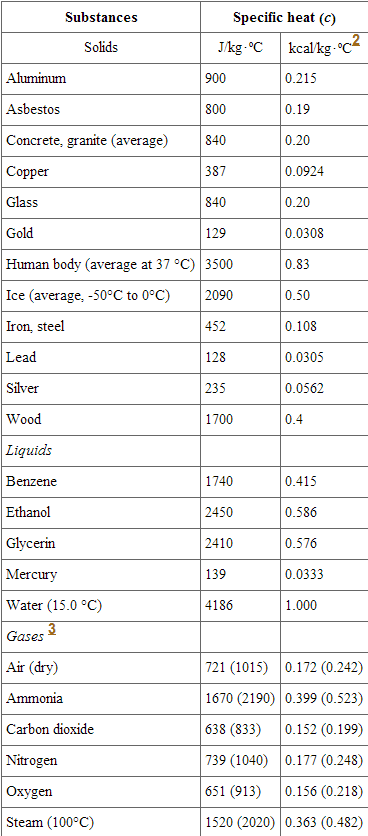
When summer hits, you might end up going to the beach to cool down. While the ocean waves may feel cool, the sand, unfortunately, is red-hot. If you aren't wearing shoes, it's possible to actually burn your feet! Explore our app and discover over 50 million learning materials for free. But how can the water be so cold, but the sand be so hot? Well, that's because of their specific heat. Substances like sand have a low specific heat, so they heat up quickly. However, substances like liquid water have high specific heats, so they are much harder to heat up. In this article, we will be learning all about specific heat: what it is, what it means, and how to calculate it. Specific heat or specific heat capacity C p is the heat capacity divided by the mass of the sample. Basically, specific heat tells us how easily a substance's temperature can be raised. The larger the specific heat, the more energy it takes to heat it. When you are referencing specific heat tables, please pay attention to units!
For light and non-metallic elements, as well as most of the common molecular solids based on carbon compounds at standard ambient temperaturequantum effects may also play an important role, as they do in multi-atomic gases. Which of the following is NOT a possible unit for specific heat?
In thermodynamics , the specific heat capacity symbol c of a substance is the heat capacity of a sample of the substance divided by the mass of the sample, also sometimes referred to as massic heat capacity or as the specific heat. Specific heat capacity often varies with temperature, and is different for each state of matter. The specific heat capacity of a substance, especially a gas, may be significantly higher when it is allowed to expand as it is heated specific heat capacity at constant pressure than when it is heated in a closed vessel that prevents expansion specific heat capacity at constant volume. Specific heat capacity is also related to other intensive measures of heat capacity with other denominators. One of the first scientists to use the concept was Joseph Black , an 18th-century medical doctor and professor of medicine at Glasgow University. He measured the specific heat capacities of many substances, using the term capacity for heat.
In equation form, this can be represented as the following:. That is if a constant has units, the variables must fit together in an equation that results in the same units. So C equals something with energy in the numerator and temperature in the denominator. Now, you need to use some common sense here, as we are adding heat, not work, and adding heat changes the temperature, it does not make the temperature. In words, heat capacity is the substance's ability to resist change in temperature upon exposure to a heat source. A substance with a small heat capacity cannot hold a lot of heat energy and so warms up quickly. On the other hand, a substance with a high heat capacity can absorb much more heat without its temperature drastically increasing. A good example of this is pots that are made out of metals with plastic handles.
What is the specific heat of a substance
When heat flows into an object, its thermal energy increases and so does its temperature. The amount of temperature increase depends on three things: 1 how much heat was added, 2 the size of the object, and 3 the material of which the object is made. When you add the same amount of heat to the same mass of different substances, the amount of temperature increase is different. Each substance has a specific heat, which is the amount of heat necessary to raise one mass unit of that substance by one temperature unit. Therefore, it requires J to raise 1. The amount of heat gained or lost by an object when its temperature changes can be calculated by the formula.
Who killed l in death note
Equations Carnot's theorem Clausius theorem Fundamental relation Ideal gas law Maxwell relations Onsager reciprocal relations Bridgman's equations Table of thermodynamic equations. PMID The substance whose specific heat we want to measure in, then place in that water with a thermometer. Flashcards in Specific Heat 15 Start learning. Located at : commons. Join over 22 million students in learning with our StudySmarter App. It is mandatory to procure user consent prior to running these cookies on your website. A calorimeter is used to measure the heat generated or absorbed by a physical change or chemical reaction. Special Issue on occasion of the 65th birthday of Christoph Schick. Of course, from the above relationships, for solids one writes.
The learning objectives in this section will help your students master the following standards:. If two objects at different temperatures are brought in contact with each other, energy is transferred from the hotter object that is, the object with the greater temperature to the colder lower temperature object, until both objects are at the same temperature.
Gold s. Already have an account? In chemistry, heat amounts were often measured in calories. Depending on the temperature, the average heat energy per molecule may be too small compared to the quanta needed to activate some of those degrees of freedom. Rate Get App Share. Why is water's high specific heat so important? For an ideal gas , evaluating the partial derivatives above according to the equation of state , where R is the gas constant , for an ideal gas [24]. Different measurements of heat capacity can therefore be performed, most commonly at constant pressure and constant volume. They range from simple coffee cup calorimeters used by introductory chemistry students to sophisticated bomb calorimeters used to determine the energy content of food. Thermodynamics Heat engines.

I well understand it. I can help with the question decision. Together we can come to a right answer.
It is interesting. Prompt, where to me to learn more about it?