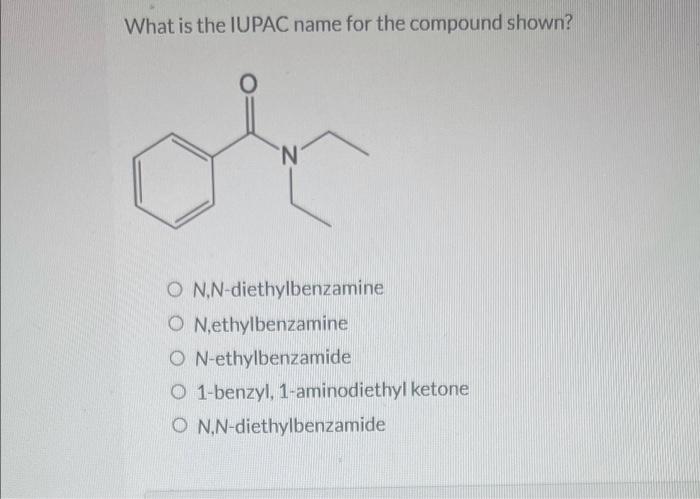
What is the iupac name for the compound shown
In order to give compounds a name, certain rules must be followed. This is to give consistency to the names. It also enables every compound to have a unique name, which is not possible with the common names used for example in industry.
In order to name organic compounds you must first memorize a few basic names. These names are listed within the discussion of naming alkanes. In general, the base part of the name reflects the number of carbons in what you have assigned to be the parent chain. The suffix of the name reflects the type s of functional group s present on or within the parent chain. Other groups which are attached to the parent chain are called substituents. Alkanes - saturated hydrocarbons The names of the straight chain saturated hydrocarbons for up to a 12 carbon chain are shown below. The names of the substituents formed by the removal of one hydrogen from the end of the chain is obtained by changing the suffix - ane to - yl.
What is the iupac name for the compound shown
.
As there are only single bonds between the carbon atoms, the prefix becomes propan.
.
We will only use those common names listed under Objective 3, above. We will use systematic names in all other cases. For example, the systematic name of the compound shown below is benzenecarbaldehyde, but it has the common name of benzaldehyde. The most potent and varied odors are aldehydes. Ketones are widely used as industrial solvents. Aldehydes and ketones contain the carbonyl group. Aldehydes are considered the most important functional group. They are often called the formyl or methanoyl group. Aldehydes derive their name from the dehyd ration of al cohols. Aldehydes contain the carbonyl group bonded to at least one hydrogen atom.
What is the iupac name for the compound shown
One way of checking whether the name you have given to an alkane is reasonable is to count the number of carbon atoms implied by the chosen name. If you were to check the given structure and find 11 carbon atoms, you would know that you had made a mistake. When naming alkanes, a common error of beginning students is a failure to pick out the longest carbon chain. Remember that every substituent must have a number, and do not forget the prefixes: di, tri, tetra, etc. You must use commas to separate numbers, and hyphens to separate numbers and substituents. Hydrocarbons having no double or triple bond functional groups are classified as alkanes or cycloalkanes , depending on whether the carbon atoms of the molecule are arranged only in chains or also in rings. Although these hydrocarbons have no functional groups, they constitute the framework on which functional groups are located in other classes of compounds, and provide an ideal starting point for studying and naming organic compounds. The alkanes and cycloalkanes are also members of a larger class of compounds referred to as aliphatic.
Pouched animal crossword clue
Find the longest continuous carbon chain that contains the functional group it won't always be a straight chain and count the number of carbon atoms in this chain. Note that the way we number the carbon atoms is important. Using atomic model kits build the molecules of methanoic acid, ethanoic acid, butanoic acid, pentanoic acid and octanoic acid. The prefix prop- tells us there are three carbon atoms in the longest chain. The prefix for this compound will be but-. Number the carbons in the longest chain In this example, you will need to number the carbons from right to left so that the triple bond is between carbon atoms with the lowest numbers the suffix for the compound will therefore be yne. The functional group is an alkane, so the suffix is -ane. There are no branched groups in this compound. This compound has the suffix -ane, but also contains a halogen atom. Give the structural representation for the following:. Combine the elements of the name into a single word in the following order: halogen atoms in alphabetical order; prefix; name ending according to functional group The name of the compound is 1,3-dibromofluorobutane. Some examples are given at the end of the list. There is a hydroxyl group, therefore the compound is an alcohol and the suffix is -ol. There are seven carbon atoms in the longest chain so the prefix is hept-. When both double bonds and carbonyl groups are present, the -en suffix follows the parent chain directly and the -al suffix follows the -en suffix notice that the e is left off, -en instead of -ene.
IUPAC nomenclature is used for the naming of chemical compounds, based on their chemical composition and their structure. Another entity called the International Association of Chemical Societies IACS existed, and on , gave vital propositions the new one should address: [2]. In , a group of chemists created the IUPAC with this idea, as well as the purpose of unionizing scientists and strengthening the international trade of science.
Look for any branched groups There are no branched groups in this compound. In this case, it doesn't matter whether we number the carbons from the left to right, or from the right to left. The compound will have the suffix -ol. There is one branched group which is a methyl group and this is at position 2. The triple bond is between the third and fourth carbon atoms regardless of how you number the chain yne. There is a carbonyl group and a hydroxyl group attached to the same carbon atom. The prefix di- is used if both alkyl groups are the same. There is a carbonyl group and it is not on the first or last carbon atom. Compare this to butyl butanoate. Identify the functional group This compound has the suffix -ane, but also contains a halogen atom. Note that if we had numbered from the right to left the suffix would still have been 1,3-diene, however the ethyl group would have been on the third carbon. In summary, the name of the compound is written out with the substituents in alphabetical order followed by the base name derived from the number of carbons in the parent chain. The double bond occurs between the first and second carbon atoms.

I think, that you commit an error. Let's discuss it.
I do not know.
Absolutely with you it agree. In it something is and it is excellent idea. It is ready to support you.