What is the difference between ionic and covalent bonds quizlet
For more option use Advanced Search. Ionic compounds, such as sodium chloride NaClare formed by a transfer of electrons that creates ions.
There are two types of atomic bonds - ionic bonds and covalent bonds. They differ in their structure and properties. Covalent bonds consist of pairs of electrons shared by two atoms, and bind the atoms in a fixed orientation. Whether two atoms can form a covalent bond depends upon their electronegativity i. This results in a positively charged ion cation and negatively charged ion anion. The bond between these two ions is called an ionic bond.
What is the difference between ionic and covalent bonds quizlet
.
The two ions are attracted to each other and form an ionic bond. Thank you!
.
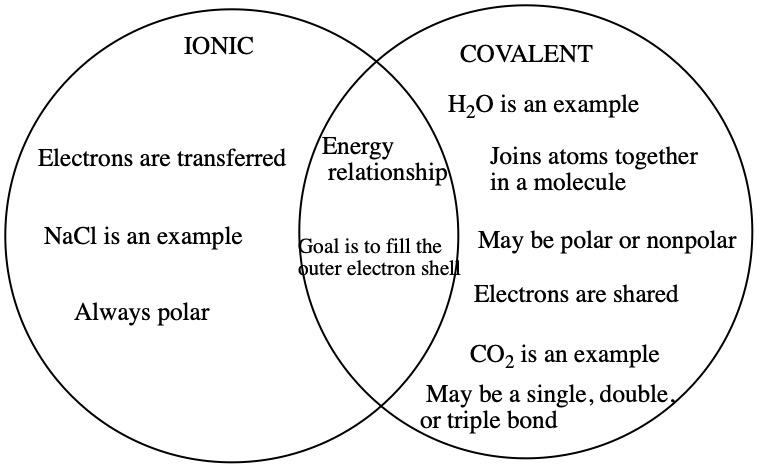
Ionic and covalent bonds are the two main types of chemical bonding. A chemical bond is a link formed between two or more atoms or ions. The main difference between ionic and covalent bonds is how equally the electrons are shared between atoms in the bond. Here is an explanation of the difference between ionic and covalent bonds, examples of each bond type, and a look at how to tell which type of bond will form. In an ionic bond, one atom donates an electron to another atom. This stabilizes both atoms. Because one atom essentially gains an electron and the other loses it, an ionic bond is polar. In other words, one atom in the bond has a positive charge, while the other has a negative charge.
What is the difference between ionic and covalent bonds quizlet
In BIS2A, we focus primarily on three different bond types: ionic bonds , covalent bonds , and hydrogen bonds. We expect students to be able to recognize each different bond type in molecular models. In addition, for commonly seen bonds in biology, we expect student to provide a chemical explanation, rooted in ideas like electronegativity, for how these bonds contribute to the chemistry of biological molecules. Ionic bonds are electrostatic interactions formed between ions of opposite charges. The origins of these interactions may arise from the association of neutral atoms whose difference in electronegativities is sufficiently high.
Pixiz text
The most common gas in the atmosphere, nitrogen, is made of two nitrogen atoms bonded by a triple bond. Water, a liquid composed of covalently bonded molecules, can also be used as a test substance for other ionic and covalently compounds. Ionic Bonds. These are mostly gaseous and even a slight negative or positive charge at opposite ends of a covalent bond gives them molecular polarity. On the other hand, Sodium has one valence electron and it also needs eight electrons. They differ in their structure and properties. As water evaporates, the salt solution becomes more and more concentrated. Each nitrogen atom is able to share three electrons for a total of six shared electrons in the N 2 molecule Fig. These kinds of bonds occur mainly between a metallic and a non metallic atom. Neither atom is "strong" enough to attract electrons from the other. Image courtesy of Tomihahndorf from Wikipedia. Sodium and chloride ions in A a large crystal, B dissolved in water as smaller crystals, and C dissociated in water. Most of them are soluble in water but insoluble in non-polar solvents. Polyatomic ions bond with other ions in the same way that elemental ions bond, with electrostatic forces caused by oppositely charged ions holding the ions together in an ionic compound bond.
It has long been known that pure carbon occurs in different forms allotropes including graphite and diamonds. This molecule was named after the architect and inventor R. Buckminster Fuller — , whose signature architectural design was the geodesic dome, characterized by a lattice shell structure supporting a spherical surface.
I think this is a really good site. Similarly, the number of electrons in the valence shell also determines ion formation. MS-PS Develop models to describe the atomic composition of simple molecules and extended structures. Image caption. To fill its valence shell, oxygen needs two additional electrons, and hydrogen needs one. Ionic bond, also known as electrovalent bond is a type of bond formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Both puppies share both bones Fig. Sodium and chlorine bonding ionically to form sodium chloride. This really helped me on my Covalent and Ionic Bonding worksheet! Covalent bonding is the type of bond that holds together the atoms within a polyatomic ion. Puppies demonstrating covalent bonding A The two puppies represent atoms, their bones represent one of their electrons. Hydrogen can participate in both ionic and covalent bonding. In general, ionic bonds occur between elements that are far apart on the periodic table.

Dismiss me from it.
Curious topic
It absolutely not agree with the previous message