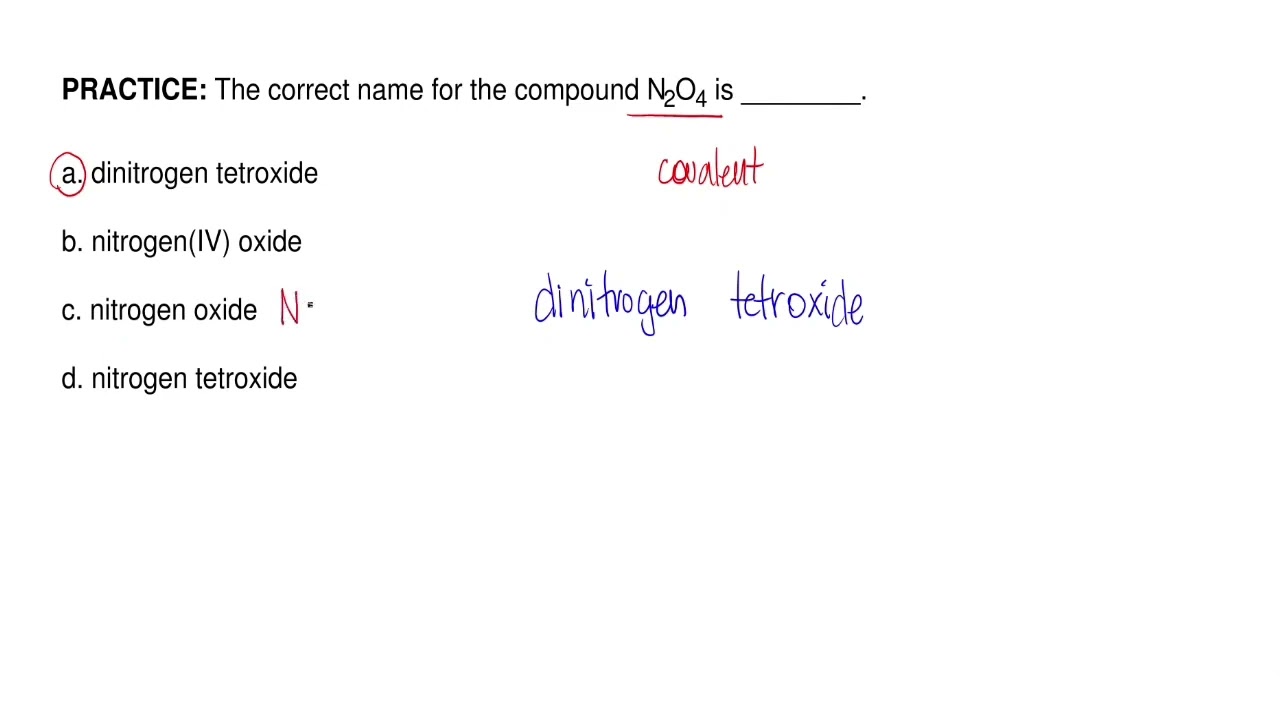
What is the correct name for n2o4
The Dinitrogen tetroxide molecule contains a total of 6 atom s.
It is a useful reagent in chemical synthesis. It forms an equilibrium mixture with nitrogen dioxide. Its molar mass is Dinitrogen tetroxide is a powerful oxidizer that is hypergolic spontaneously reacts upon contact with various forms of hydrazine , which has made the pair a common bipropellant for rockets. Dinitrogen tetroxide could be regarded as two nitro groups -NO 2 bonded together.
What is the correct name for n2o4
.
Oxford University Press. Tools Tools.
.
It is a useful reagent in chemical synthesis. It forms an equilibrium mixture with nitrogen dioxide. Its molar mass is Dinitrogen tetroxide is a powerful oxidizer that is hypergolic spontaneously reacts upon contact with various forms of hydrazine , which has made the pair a common bipropellant for rockets. Dinitrogen tetroxide could be regarded as two nitro groups -NO 2 bonded together. The N-N distance corresponds to a weak bond, since it is significantly longer than the average N-N single bond length of 1. Unlike NO 2 , N 2 O 4 is diamagnetic since it has no unpaired electrons. Higher temperatures push the equilibrium towards nitrogen dioxide. Inevitably, some dinitrogen tetroxide is a component of smog containing nitrogen dioxide. Nitrogen tetroxide is made by the catalytic oxidation of ammonia : steam is used as a diluent to reduce the combustion temperature.
What is the correct name for n2o4
It is colorless in solid form while in liquid and gaseous form; it has a characteristic reddish-brown color. N2O4 exists in equilibrium with NO2 in both liquid and gaseous forms. Therefore, it is regarded as two NO2 groups that are bonded together. The reddish-brown color of the liquid form is due to the presence of the NO2 group. Nitrogen tetroxide is a strong oxidizer.
Flats to rent in liverpool
Its molar mass is Chemical Formula Description The Dinitrogen tetroxide molecule contains a total of 6 atom s. BBC News in Spanish. Infobox references. The law of conservation of mass dictates that the quantity of each element given in the chemical formula does not change in a chemical reaction. Dinitrogen tetroxide can also be produced by heating metal nitrates. This hot nitrogen dioxide is expanded through a turbine, cooling it and lowering the pressure, and then cooled further in a heat sink, causing it to recombine into nitrogen tetroxide at the original molecular weight. Additionally, NTO is often used with the addition of a small percentage of nitric oxide , which inhibits stress-corrosion cracking of titanium alloys, and in this form, propellant-grade NTO is referred to as mixed oxides of nitrogen MON. Inevitably, some dinitrogen tetroxide is a component of smog containing nitrogen dioxide. Such compounds must be prepared in anhydrous conditions, since the nitrate ion is a much weaker ligand than water, and if water is present the simple nitrate of the hydrated metal ion will form. Most of the water is condensed out, and the gases are further cooled; the nitric oxide that was produced is oxidized to nitrogen dioxide, which is then dimerized into nitrogen tetroxide:. New York: Reinhold. The tendency of N 2 O 4 to reversibly break into NO 2 has led to research into its use in advanced power generation systems as a so-called dissociating gas. Related nitrogen oxides. Confirm Valid email address confirmed.
Explore more content.
Potential performance improvement by using a reacting gas nitrogen tetroxide as the working fluid in a closed Brayton cycle PDF Technical report. J: Prentice Hall. It is then much easier to compress to start the entire cycle again. In early , research on the usability of dinitrogen tetroxide as an oxidizing agent for rocket fuel was conducted by German scientists, although the Germans only used it to a very limited extent as an additive for S-Stoff fuming nitric acid. This hot nitrogen dioxide is expanded through a turbine, cooling it and lowering the pressure, and then cooled further in a heat sink, causing it to recombine into nitrogen tetroxide at the original molecular weight. Germany Israel United States Japan. Descriptive inorganic chemistry 6th ed. Refractive index n D. Nitrogen tetroxide is used as an oxidizing agent in one of the most important rocket propellant systems because it can be stored as a liquid at room temperature. When used as a propellant, dinitrogen tetroxide is usually referred to simply as nitrogen tetroxide and the abbreviation NTO is extensively used.

0 thoughts on “What is the correct name for n2o4”