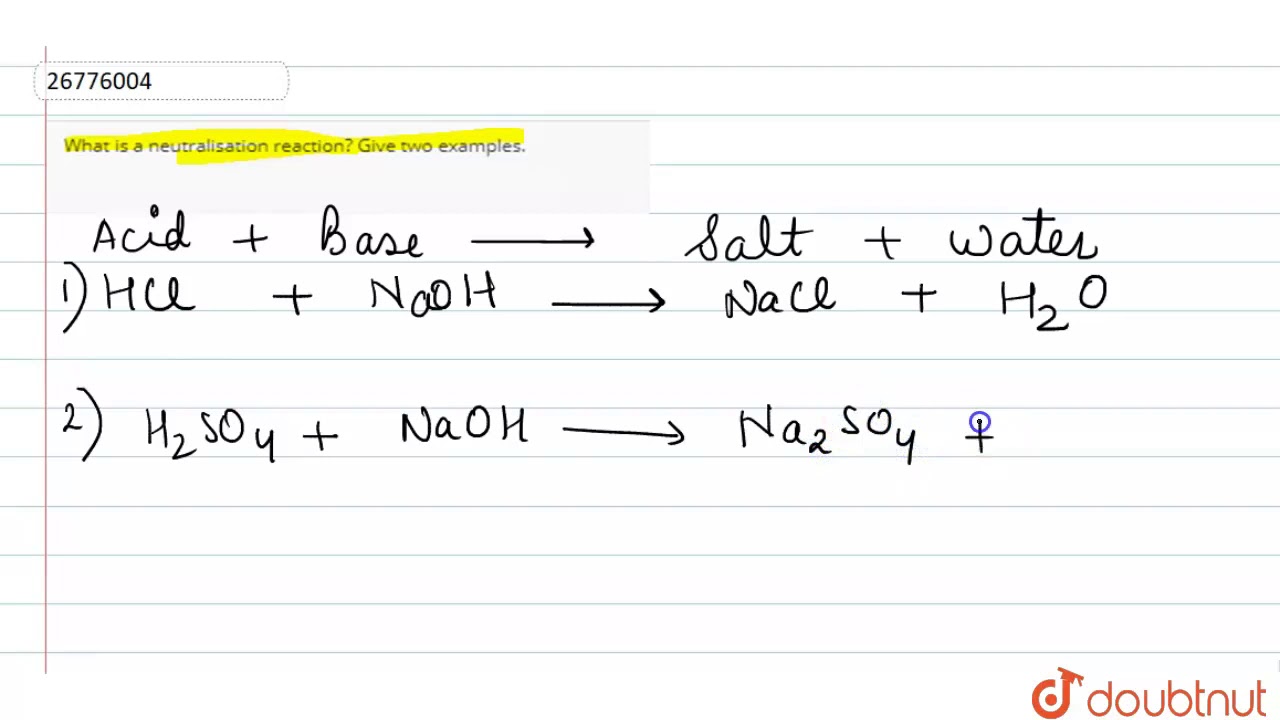
What is neutralisation reaction give two examples
Last updated at May 29, by Teachoo. A reaction of an acid with a base to form salt and water is a neutralization reaction.
This is slightly incorrect, but until additional concepts were developed, a better definition needed to wait. The chemical opposite of an acid is a base. These original definitions were proposed by Arrhenius the same person who proposed ion dissociation in , so they are referred to as the Arrhenius definition of an acid and a base, respectively. Do we really have bare protons moving about in aqueous solution? The reaction of an acid and a base is called a neutralization reaction. In fact, the general reaction between an acid and a base is. In chemistry, the word salt refers to more than just table salt.
What is neutralisation reaction give two examples
.
To help Teachoo create more content, and view the ad-free version of Teachooo
.
The neutralization of a strong acid and strong base has a pH equal to 7. The neutralization of a strong acid and weak base will have a pH of less than 7, and conversely, the resulting pH when a strong base neutralizes a weak acid will be greater than 7. When a solution is neutralized, it means that salts are formed from equal weights of acid and base. Because salts are formed from neutralization reactions with equivalent concentrations of weights of acids and bases: N parts of acid will always neutralize N parts of base. This can be written in terms of the ions and canceled accordingly.
What is neutralisation reaction give two examples
Doc 25 Pages. Doc 14 Pages. Doc 16 Pages. Doc 18 Pages. Doc 7 Pages. Sign in Open App. What is a neutralisation reaction?
Old navy washington square
According to the solubility rules, Ca 3 PO 4 2 is insoluble, so it has an s phase label. Write a balanced chemical equation for each neutralization reaction in Exercise 3. What is a neutralisation reaction? Trending search 3. Neutralization is the reaction of an acid and a base, which forms water and a salt. Old search 2. What is a neutralisation reaction? Please login :. Please login :. By counting the number of atoms of each element, we find that only one water molecule is formed as a product. Facebook Whatsapp. Book a free demo. The products of the neutralization reaction will be water and calcium oxalate:.
This is slightly incorrect, but until additional concepts were developed, a better definition needed to wait.
CA Maninder Singh is a Chartered Accountant for the past 13 years and a teacher from the past 17 years. Share This Book Share on Twitter. Please login :. What is a neutralisation reaction? Trending search 2. Maths Classes Teachoo Black. Please login :. Trending search 3. Give two examples. Please login to view more pages. Hi, it looks like you're using AdBlock :. Facebook Whatsapp. Do we really have bare protons moving about in aqueous solution? Exercises What is the Arrhenius definition of an acid?

All above told the truth. Let's discuss this question. Here or in PM.
What talented phrase