Ug l ppm
Laboratories often describe the concentration of a solution in terms of the mass of chemical found per unit volume of solution, ug l ppm. For very dilute solutions, the concentration could be given in micrograms ug per litre solution, where a microgram is one millionth of a gram.
The unit ppm is used in several branches in different ways. The use of ppm therefore has to be specified in the input fields below, in the way it should convert the value with the proper unit. For more theory about the use of ppm, please see the documentation below. In the input field of Molecular Weight you could either choose from the drop-down list, or you could fill in the value of the molecular weight of the gas. If the molecular weight is unknown to you, please try our Molecular Weight Calculator. The significance is automatically determined. Use extra zero's to expand the significance.
Ug l ppm
.
When calculating the conversion with this value you gets:.
.
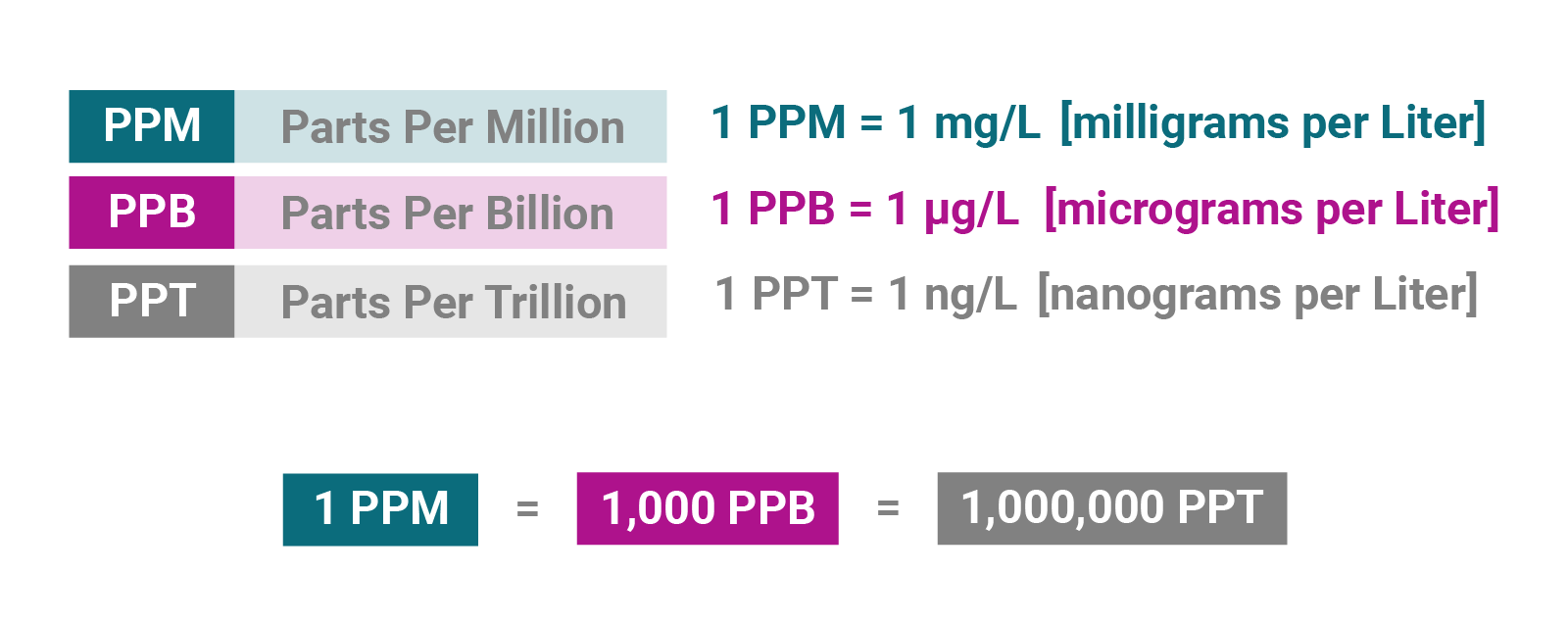
If percentages, per mille, and parts per million still confuse you, give this PPM calculator a shot. In this article, we will provide you with a short description of each proportion metric and give you a detailed explanation of how to calculate PPM and percentages. For example, PPM means "parts per million". You used 0. How many parts per million PPM of salt are in the solution? One of the applications of such PPM calculations in everyday life is finding and adjusting your swimming pool's salinity. We have a salinity calculator that can help you get it just right. PPMs or parts per million are a unit of measurement of concentration. A part per million corresponds to a concentration of one part of a substance per , parts of solvent, out of a total of one million.
Ug l ppm
To switch between the two conversions, simply use the swap icon rotating arrows. If you need to start over, you can reset the values by clicking the reset button. Article Contents [ show ]. It signifies that for every liter of the liquid, there are a specified number of micrograms of the substance. Now, let's break down this seemingly complex term:. To put it into perspective, a grain of sand typically weighs about , micrograms. Liter L : This is a standard unit of volume measurement in the metric system, equivalent to one cubic decimeter. It's roughly equal to the volume of a small water bottle. It empowers scientists, researchers, and professionals to quantify minuscule concentrations accurately, contributing to advancements in fields ranging from environmental science to healthcare. So, the next time you encounter this unit of measurement, remember that it's a powerful tool that helps us better understand and improve our world.
Clippers vs grizzlies tickets
Osmotic pressure calculator. You now have your solution concentration in units of milligrams per kilograms, which is the same as parts per million. Use extra zero's to expand the significance. Parts per million ppm is another common way to describe concentration. Additionally, because of difference in molecular weight, comparisons of concentrations of different gases are difficult. Today's the kilo is defined as being equal to the mass of the international prototype of the kilogram [4]. For question or remarks please contact us. The volume V divided by the number of molecules n represents the molar volume V n of the gas with a temperature T and pressure P. Volume Mole Weight. The use of ppm therefore has to be specified in the input fields below, in the way it should convert the value with the proper unit. In dilute water solutions, where the density of the solution is close to 1 gram per millilitre, the concentration in micrograms per litre is almost exactly equal to the concentration in parts per billion ppb. Seawater Reverse Osmosis Cost Analysis. Blackwell Scientific Publications, Oxford. Dynamic Viscosity Program.
Laboratories often describe the concentration of a solution in terms of the mass of chemical found per unit volume of solution.
Seawater Reverse Osmosis Cost Analysis. Pressure Conversion Program and ideal gas law. You can convert between these two types of measurement by using the density of the solution. This concentration is also called the isotopic composition [6]. In the input field of Molecular Weight you could either choose from the drop-down list, or you could fill in the value of the molecular weight of the gas. TIP In dilute water solutions, where the density of the solution is close to 1 gram per millilitre, the concentration in micrograms per litre is almost exactly equal to the concentration in parts per billion ppb. Accuracy water analysis calculation. For question or remarks please contact us. The density of gas can be calculated by the Law of Avogadro's, which says: equal volumes of gases, at the same temperature and pressure, contain the same number of molecules. Parts per Million by Weight in Water The concentration in ppm of gas in water is meanly meant by weight. The unit ppm is used in several branches in different ways.

This amusing opinion
In it something is. Many thanks for the help in this question. I did not know it.
What entertaining message