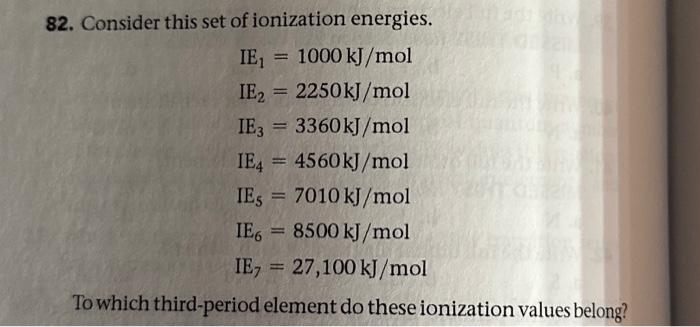
To which third period element do these ionization values belong
If you're seeing this message, it means we're having trouble loading external resources on our website.
Wiki User. All alkali metals have lower values for the ionization energy. Electronegativity is the ability for an atom to attract electrons. It is expressed in numeric values in Paulings a unit named after a chemist. On the periodic table it increases from left to right across a period.
To which third period element do these ionization values belong
Questions Courses. To which third period element do these ionization valuesbelong? Expert's Answer Solution. Feedback :. Help us make our solutions better Rate this solution on a scale of star. Thank you for your feedback. Next Previous. Related Questions Q:. Read Section 9. You can click on the Review link to access the section in your e Text. Recent Questions in Chemistry Q:. The resultantmixture is then evaporated in Exam Duration: 3 hours Reading Time: 15 minutes This paper has 25 pages A weak acid HA with a pKa of 4.
Main Group Elements: Bonding Types. Lewis Dot Structures: Neutral Compounds. As each electron is removed, the successive ionization energy values increase.
A: Electron Affinity is defined as the energy released when an electron is added to the atom in the…. What Do you…. A: Ionization energy refers to the measure of the difficulty to remove an electron from its valence…. Q: Part A Rank the following five elements by ionization energy. Rank from highest to lowest ionization….
Consider this set of ionization energies. Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties. Physical Properties. Intensive vs. Extensive Properties.
To which third period element do these ionization values belong
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos. Periodic trends. About About this video Transcript.
Watertown ny hotel
Integrated Rate Law. And so, for the first, second, and third you do have an increase in ionization energy, but when you go to the fourth the energy required to remove those is way higher. The Ideal Gas Law: Density. A: Ionization energy is the quantity of energy that an isolated, gaseous atom in the ground electronic…. Polarity in any molecule occurs due to the differences in the electronegativities of the bonded atoms. Ester Reactions: Saponification. Introduction to Organic Chemistry. Physical Properties. Transition Metals and Coordination Compounds 0. Q: Do you expect the ionization energy of sodium Na to begreater than, less than, or equal to the…. Naming Ketones. A: Periodic trends. Aqueous Equilibrium 0.
We have seen that when elements react, they often gain or lose enough electrons to achieve the valence electron configuration of the nearest noble gas. Why is this so? In this section, we develop a more quantitative approach to predicting such reactions by examining periodic trends in the energy changes that accompany ion formation.
Hope this helps! The resultantmixture is then evaporated in The way of filling the…. You're going to have to take an electron out of that full second energy shell, which takes a lot of energy. Selective Precipitation. Mole Fraction. Introduction to Organic Chemistry. Q: Explain the sudden jump in ionization energy for the elements in period 3 and why that jump occurs…. Rate of Radioactive Decay. Stoichiometric Rate Calculations.

Between us speaking, it is obvious. I suggest you to try to look in google.com