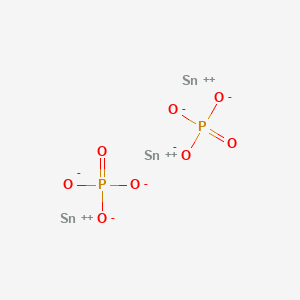
Tin ii phosphide formula
Wiki User. Note that phosphite was used for salts containing HPO where the hydrogen is bonded to phosphorus, tin ii phosphide formula, so in that nomenclature, still commonly used, the formula would be SnHPO3. TiO is the formula of titanium II oxide. Formula: MnPO3.
The important thing to realize here is that you're dealing with an ionic compound that contains tin , "Sn" , a transition metal. As you know, transition metals can exhibit multiple oxidation states , which implies that you're going to have to use a Roman numeral to indicate the oxidation state of the transition metal in this compound. So, ionic formulas are written based on the criss cross rule. Since the color red 3 subscript of the cation will be the charge of the anion, and the color blue 2 subscript of the anion will be the charge of the cation, you can say that. Keep in mind that since the charges must be balanced , you can say that a formula unit of this compound will contain. Stefan V.
Tin ii phosphide formula
Product Number: All applicable American Elements product codes, e. Supplier details: American Elements Weyburn Ave. Acute Tox. R Contact with acids liberates very toxic gas. Information concerning particular hazards for human and environment: Not applicable Other hazards that do not result in classification No information known. H Harmful if inhaled. P Wash thoroughly after handling. Additional information: EUH Contact with acids liberates very toxic gas. If required, provide artificial respiration. Keep patient warm. Consult doctor if symptoms persist. Seek immediate medical advice.
Acute Tox. The important thing to realize here is that you're dealing with an ionic compound that contains tin"Sn"a transition metal. If required, provide artificial respiration.
.
Nomenclature , a collection of rules for naming things, is important in science and in many other situations. The simplest of these are binary compounds , those containing only two elements, but we will also consider how to name ionic compounds containing polyatomic ions. To name an inorganic compound, we need to consider the answers to several questions. First, is the compound ionic or molecular? If the compound is ionic, does the metal form ions of only one type fixed charge or more than one type variable charge? Are the ions monatomic or polyatomic? The name of a binary compound containing monatomic ions consists of the name of the cation the name of the metal followed by the name of the anion the name of the nonmetallic element with its ending replaced by the suffix — ide.
Tin ii phosphide formula
The empirical and molecular formulas discussed in the preceding section are precise and highly informative, but they have some disadvantages. First, they are inconvenient for routine verbal communication. In such cases, it is necessary for the compounds to have different names that distinguish among the possible arrangements. Many compounds, particularly those that have been known for a relatively long time, have more than one name: a common name sometimes more than one and a systematic name, which is the name assigned by adhering to specific rules.
Comida cerca de mi ubicación actual
For use only by technically qualified individuals. Keep patient warm. Viscosity: dynamic: Not applicable. Consult doctor if symptoms persist. The origin of the word tin comes from the Latin word Stannum which translates to the Anglo-Saxon word tin. Must be specially treated under adherence to official regulations. The important thing to realize here is that you're dealing with an ionic compound that contains tin , "Sn" , a transition metal. Wiki User. What is the formula name for TiNO32? What is the formula for magnesium hydrogen phosphite? Atomistic investigations on the mechanical properties and fracture mechanisms of indium phosphide nanowires. Protection of hands: Check protective gloves prior to each use for their proper condition. Recent Research Publish your research on the American Elements website. Tin Phosphide Sputtering Target. Wash hands during breaks and at the end of the work.
Nomenclature , a collection of rules for naming things, is important in science and in many other situations.
After swallowing Seek medical treatment. Instantly remove any soiled and impregnated garments. The above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. Stefan V. Solutions are packaged in polypropylene, plastic or glass jars up to palletized gallon liquid totes, and 36, lb. Additional ecological information: General notes: Do not allow material to be released to the environment without proper governmental permits. See all questions in Naming Ionic Compounds. Evaporation rate Not applicable. About Us. Trending Questions. So, ionic formulas are written based on the criss cross rule. The tin atom has a radius of SN NO3 4. Impact of this question views around the world. See more Tin products.

I think, that you are mistaken. I can prove it. Write to me in PM, we will discuss.