The triple bond in ethyne is made up of
Finally, the hybrid orbital concept applies well to triple-bonded groups, such as alkynes and nitriles. Consider, for example, the structure of ethyne another common name is acetylenethe simplest alkyne. This molecule is linear: all four atoms lie in a straight line. The carbon-carbon triple bond is only 1.
We know that the building block of structural organic chemistry is the tetravalent carbon atom. With some exceptions, carbon compounds can be formulated with four covalent bonds with carbon or some other element. The two-electron bond, which is shown by the carbon-hydrogen bonds in methane or ethane and the carbon-carbon bond in ethane, is called a single bond. In these and many similar substances, each carbon is attached to four other atoms as:. There are compounds such as ethene ethylene , C2H4, in which two electrons from each of the carbon atoms are mutually shared, producing two two-electron bonds, an arrangement which is called a double bond. Each carbon in ethene is attached to three other atoms as:.
The triple bond in ethyne is made up of
Three sigma bond. Three pi bond. One signal bond and two pi bonds. Two sigma and one pi bonds. The triple bond in ethyne is made up of. The triple bond in ethyne is made of. The triple bond in carbon monoxide consists of :. Water can be added across a triple bond in the presence of. The organic compounds having double or triple bonds in them are termed as ………….. One hybridization of one s and one p orbital we get. If molecule MX3 has zero dipole moment, the sigma bonding orbitals use The melecule that has linear structure is:.
A triple bond is made up of a sigma bond and two pi bonds. It is possible to have hybridization in three different ways.
Acetylene is the simplest member of the alkyne family. Alkynes are unsaturated hydrocarbons in which a carbon-carbon triple bond exists between the two carbon atoms. Quantum mechanics helps us a great deal to study the structure of different molecules found in nature. The concept of chemical bonding in combination with quantum mechanics has revealed numerous information about various organic and inorganic compounds that are essential for life. This article deals with the structure of a special class of organic compounds known as alkynes. When three pairs of electrons are shared between two carbon atoms, a triple bond is formed between the two carbon atoms.
Structure of Triple Bond: What does triple bond mean? What is the structure of triple bond and what significance does it hold? We will try to answer to the above questions and others through this article. Verify OTP Code required. I agree to the terms and conditions and privacy policy. First name. Last name.
The triple bond in ethyne is made up of
Hybridization was introduced to explain molecular structure when the valence bond theory failed to correctly predict them. It is experimentally observed that bond angles in organic compounds are close to o , o , or o. According to Valence Shell Electron Pair Repulsion VSEPR theory, electron pairs repel each other and the bonds and lone pairs around a central atom are generally separated by the largest possible angles. Carbon is a perfect example showing the value of hybrid orbitals. Carbon's ground state configuration is:.
Volvo p1800 price in india
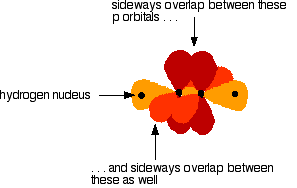
Ethyneeis built from hydrogen atoms 1s1 and carbon atoms 1s22s22px12py1. What is Olympiad Exam? The C-C sigma bond is formed by the overlap of one sp orbital from each of the carbons, while the two C-H sigma bonds are formed by the overlap of the second sp orbital on each carbon with a 1s orbital of a hydrogen atom. Some diatomic molecules, such as di nitrogen and carbon monoxide, are also triple bonded. It is also used for making polymers and its initiating materials. The number of valence electrons in carbon atom is. The ethyne molecule has a triple bond formed by two carbon atoms, each of which is singly bonded to one hydrogen atom. Acetylene is a straight-line molecule with a carbon-carbon distance of 1. Because it contains a carbon-carbon triple bond, ethyne or acetylene is an unsaturated hydrocarbon. The two-electron bond, which is shown by the carbon-hydrogen bonds in methane or ethane and the carbon-carbon bond in ethane, is called a single bond.
Finally, the hybrid orbital concept applies well to triple-bonded groups, such as alkynes and nitriles.
For the bond to form well, there has to be a proper geometrical relationship between the unhybridized p orbitals: they must be in the same plane. The C-C sigma bond is formed by the overlap of one sp orbital from each of the carbons, while the two C-H sigma bonds are formed by the overlap of the second sp orbital on each carbon with a 1s orbital of a hydrogen atom. Alkynes are nonpolar, since they contain nothing other than carbon and hydrogen, and so, like the alkanes and alkenes, they are insoluble in water, and are usually less dense than water. Consider, for example, the structure of ethyne another common name is acetylene , the simplest alkyne. The triple-bonded carbon atoms of acetylene are sp hybridized. In this chapter we will discuss zwitterion,characteristics of zwitterion, isoelectric point, pH value, and application. Coordinate linkage is formed. Millikan Oil Drop Experiment. Graphite Structure. Ethyne, hence, has three sigma bonds and two pi bonds. Alkynes are usually used to artificially ripe fruits. The orbital structure of ethyne, as shown in the figure below, illustrates the structure of the triple bond:. Share Share Share Call Us. The carbon atom does not have enough unpaired electrons to form four bonds 1 to the hydrogen and three to the other carbon , so it has to promote one of the 2s2 pair into the vacant 2pz orbital. Watch Now.

I recommend to you to look for a site where there will be many articles on a theme interesting you.
In my opinion you are mistaken. Let's discuss it. Write to me in PM, we will communicate.