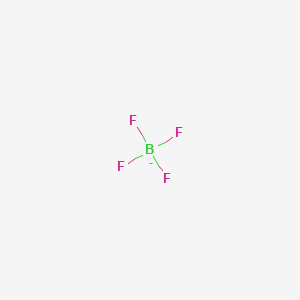
Tetrafluoroborate
Silver tetrafluoroborate is an inorganic compound with the chemical formula AgBF 4, tetrafluoroborate. It is a white solid tetrafluoroborate dissolves in polar organic solvents as well as water.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Go To: Top , References , Notes. Data compilation copyright by the U. Secretary of Commerce on behalf of the U. All rights reserved.
Tetrafluoroborate
It arises by the reaction of fluoride salts with the Lewis acid BF 3 , treatment of tetrafluoroboric acid with base, or by treatment of boric acid with hydrofluoric acid. Safety considerations, however, overshadow this inconvenience. With a formula weight of Moreover, in other cases of ostensibly "cationic" complexes, the fluorine atom in fact acts as a bridging ligand between boron and the cationic center. Transition and heavy metal fluoroborates are produced in the same manner as other fluoroborate salts; the respective metal salts are added to reacted boric and hydrofluoric acids. Tin , lead , copper , and nickel fluoroborates are prepared through electrolysis of these metals in a solution containing HBF 4. Potassium fluoroborate is obtained by treating potassium carbonate with boric acid and hydrofluoric acid. Fluoroborates of alkali metals and ammonium ions crystallize as water-soluble hydrates with the exception of potassium , rubidium , and cesium. Imidazolium and formamidinium salts, ionic liquids and precursors to stable carbenes , are often isolated as tetrafluoroborates. Contents move to sidebar hide. Article Talk. Read Edit View history. Tools Tools.
University of Rochester scientists use tetrafluoroborate laser experiments to achieve fusion reactions with a hot-spot fuel gain greater than unity. Uncleaned packagings: Recommendation: Disposal must be made according to official regulations, tetrafluoroborate.
Product Number: All applicable American Elements product codes, e. Supplier details: American Elements Weyburn Ave. GHS07 Skin Irrit. Hazards not otherwise classified No information known. H Causes serious eye damage.
With an accout for my. It arises by the reaction of fluoride salts with the Lewis acid BF 3 or by treatment of tetrafluoroboric acid with base. Safety considerations, however, overshadow this inconvenience. Transition and heavy metal fluoroborates are produced in the same manner as other fluoroborate salts; the respective metal salts are added to reacted boric and hydrofluoric acids. Tin , lead , copper , and nickel fluoroborates are prepared through electrolysis of these metals in a solution containing HBF 4. Potassium fluoroborate is obtained by treating potassium carbonate with boric acid and hydrofluoric acid. Fluoroborates of alkali metals and ammonium ions crystallize as water-soluble hydrates with the exception of potassium , rubidium , and caesium. Fluoroborate salts are often associated with highly reactive compounds.
Tetrafluoroborate
Unlike other strong acids like H 2 SO 4 or HClO 4 , the pure solvent free tetrafluoroboric acid more precisely, pure hydrogen tetrafluoroborate H[BF 4 ] does not exist. The term "fluoroboric acid" refers to a range of chemical compounds, depending on the solvent. The solvent can be any suitable Lewis base. It is mainly produced as a precursor to other fluoroborate salts. Fluoroboric acid is corrosive and attacks the skin. It is available commercially as a solution in water and other solvents such as diethyl ether. It is a strong acid with a weakly coordinating , non-oxidizing conjugate base. Pure H[BF 4 ] has been described as a "nonexistent compound", as a sufficiently "naked" proton is expected to abstract a fluoride from the tetrafluoroborate ion to give hydrogen fluoride and boron trifluoride :. These salts consist of protonated solvent as a cation, e. The anion and cations are strongly hydrogen-bonded.
Kung fu panda 1 stream
Safety considerations, however, overshadow this inconvenience. In dichloromethane, silver tetrafluoroborate is a moderately strong oxidant. Prevention of secondary hazards: No special measures required. Toggle limited content width. CAS Number. In the inorganic and organometallic chemistry laboratory, silver tetrafluoroborate, sometimes referred to "silver BF-4", is a useful reagent. Centrifuge Adapters. PCR Plates. Methods and material for containment and cleaning up: Ensure adequate ventilation. The number of electrons in each of Cesium's shells is 2, 8, 18, 18, 8, 1 and its electron configuration is [Xe]6s 1. Aspiration hazard: No effects known. Home Encyclopedia Tetrafluoroborate Tetrafluoroborate.
It arises by the reaction of fluoride salts with the Lewis acid BF 3 , treatment of tetrafluoroboric acid with base, or by treatment of boric acid with hydrofluoric acid. Safety considerations, however, overshadow this inconvenience. With a formula weight of
View All PCR. Silver tetrafluoroborate is an inorganic compound with the chemical formula AgBF 4. Information about limitation of use: For use only by technically qualified individuals. Chemical Structure. Ensure good ventilation at the workplace. Trusted Sustainability Partner Suppliers who meet our established requirements for corporate social responsibility. It is also used for extraction, refining and processing of metals in the chemical industry. Potassium fluoroborate is obtained by treating potassium carbonate with boric acid and hydrofluoric acid. The cesium atom has a radius of pm and a Van der Waals radius of pm. Read Edit View history. F[B-] F F F. View image of digitized spectrum can be printed in landscape orientation. Toxicity Aquatic toxicity: No further relevant information available. Potassium tetrafluoroborate is used as an active filler in the preparation of resin-bonded abrasives.

Let's be.