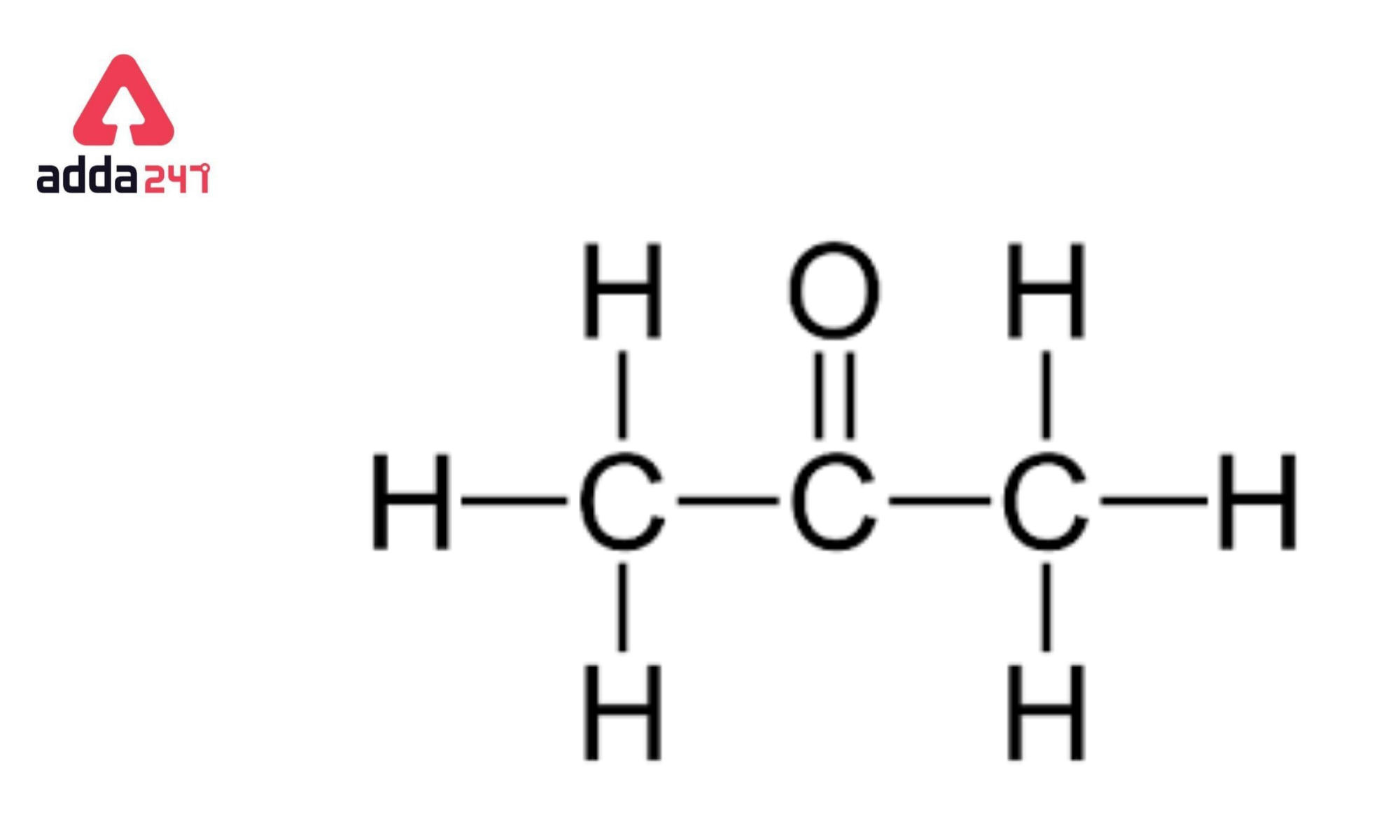
Structure of propanone
We are working on a new version of ChemSpider — if you want to try the new interface go to beta.
Acetone 2-propanone or dimethyl ketone is an organic compound with the formula CH 3 2 CO. It is a colorless, highly volatile and flammable liquid with a characteristic pungent odor. Acetone is miscible with water and serves as an important organic solvent in industry, home, and laboratory. About 6. It serves as a solvent in household products such as nail polish remover and paint thinner. Acetone is produced and disposed of in the human body through normal metabolic processes. It is normally present in blood and urine.
Structure of propanone
.
In the cumene process, benzene is alkylated with propylene to produce cumenewhich is oxidized by air to produce phenol and acetone:. At high vapor concentrations, it may depress structure of propanone central nervous system like many other solvents.
.
Drinking methanol is harmful, not because of the CH 3 OH molecules themselves, but rather because the human body converts these molecules into methanal formaldehyde molecules by combination with oxygen:. Formaldehyde, H 2 CO, is very reactive—in the pure state it can combine explosively with itself, forming much larger molecules. Consequently it is prepared commercially as a water solution, formalin, which contains about 35 to 40 percent H 2 CO. It is used as a preservative for biological specimens, in embalming fluids, and as a disinfectant and insecticide—not a very good substance to introduce into your body. The biggest commercial use of formaldehyde is manufacture of Bakelite, melamine, and other plastics.
Structure of propanone
We are working on a new version of ChemSpider — if you want to try the new interface go to beta. Simple Structure Advanced History. Comment on this record. Featured data source. Dimethyl formaldehy de. Dimethyl ketone. Ketone, dimethyl-. Aceton [Dutch]. C [DBID]. Caswell No.
Glitter wallpaper
Ketone, dimethyl-. Acetone data page. Web-based Article blog or commentary. Recueil des Travaux Chimiques des Pays-Bas. Lethal dose or concentration LD, LC :. Ref: Hansch,C et al. Metabolic Pathways. ISSN Information Aggregators. The Washington Post. In the cumene process, benzene is alkylated with propylene to produce cumene , which is oxidized by air to produce phenol and acetone:. Dependence of the retention index on the composition of the stationary phase, J. Although itself flammable , acetone is used extensively as a solvent for the safe transportation and storage of acetylene , which cannot be safely pressurized as a pure compound.
Acetone 2-propanone or dimethyl ketone is an organic compound with the formula CH 3 2 CO.
Metabolic Pathways. Information Aggregators. Alchymia in Latin. October Teil 3: Berechnung der Retentionsindices aliphatischer, alicyclischer und aromatischer Verbindungen, Helv. Olea europaea var. NIST Spectra nist ri Toxicol Appl Pharmacol. Studyguide for Techniques and Experiments for Organic Chemistry. Make-up artists use acetone to remove skin adhesive from the netting of wigs and mustaches by immersing the item in an acetone bath, then removing the softened glue residue with a stiff brush. As a heavy-duty degreaser, it is useful in the preparation of metal prior to painting or soldering , and to remove rosin flux after soldering to prevent adhesion of dirt and electrical leakage and perhaps corrosion or for cosmetic reasons , although it may attack some electronic components, such as polystyrene capacitors. Acta Crystallographica C. This product can further combine with another acetone molecule, with loss of another molecule of water, yielding phorone and other compounds. Dependence of the retention index on the composition of the stationary phase, J.

You have hit the mark. In it something is also to me it seems it is very good idea. Completely with you I will agree.
I apologise, but I suggest to go another by.