So4 lewis dot structure
The sulfate ion Sulfate Standard is a polyatomic anion with the empirical formula SO 4 Salts, acid derivatives, and peroxides of sulfate are widely used in industry, so4 lewis dot structure. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid, and many are prepared from that acid.
Lewis structures are another way to represent molecules. Lewis Structures were introduced by Gilbert N. Lewis in Lewis suggested the use of lines between atoms to indicate bonds, and pairs of dots around atoms to indicate lone or non-bonding pairs of electrons. In the example above, 3 hydrogen atoms with one valence electron each form three bonds with one nitrogen atom with 5 valence electrons. By forming three bonds, nitrogen gains 3 electrons to make a total of 8 surrounding it.
So4 lewis dot structure
Skip to main content. Table of contents. Intro to General Chemistry 0. Classification of Matter. Chemical Properties. Physical Properties. Intensive vs. Extensive Properties. Scientific Notation. Metric Prefixes. Significant Figures. Significant Figures: Precision in Measurements. Significant Figures: In Calculations. Conversion Factors. Dimensional Analysis.
Naming Alkynes. Introduction to Organic Chemistry. Chemistry of the Nonmetals 0.
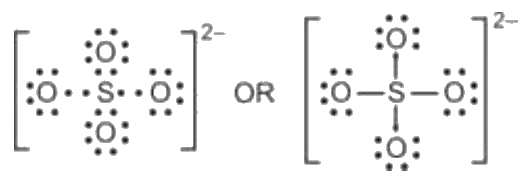
Lewis dot structure of SO 4 2 - :. Lewis Dot Structure of NO 2 - :. Byju's Answer. Open in App. Steps to draw the lewis structure: Lewis dot structures are diagrams that show the bonding between atoms of a molecule, as well as lone pairs of electrons that may exist in the molecule. First, we have to find out how many valence electrons are in the molecule.
It is usually easier to figure out a problem if you can draw a picture, either mental or real, of what is happening. This is often done in physics and mathematics, and it is especially helpful when looking at the bonding, structure, physical properties, and reactivity of compounds. The most common picture, or model, of elements and compounds used is the Lewis Dot Structure. These pictures show you the type s of atom s involved, their position in the molecule, and where their valence electrons are situated. Dash each dash represents two electrons that are shared between two atoms as a covalent bond. The valence electron configurations of the constituent atoms of a covalent compound are important factors in determining its structure, stoichiometry, and properties. For example, chlorine, with seven valence electrons, is one electron short of an octet. If two chlorine atoms share their unpaired electrons by making a covalent bond and forming Cl 2 , they can each complete their valence shell:. Each chlorine atom now has an octet.
So4 lewis dot structure
The Sulfur atom S is at the center and it is surrounded by 4 Oxygen atoms O. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of SO4 2- ion. Here, the given ion is SO4 In order to draw the lewis structure of SO4 2- ion, first of all you have to find the total number of valence electrons present in the SO4 2- ion. Valence electrons are the number of electrons present in the outermost shell of an atom. Sulfur is a group 16 element on the periodic table.
Thermaltake core v1 spcc mini itx
Place remaining electrons on the central atom, usually in pairs. The Electron Configuration: Ions. Hence, the sulfur atom S is the center atom, and the oxygen atoms O are the outside atoms. Lewis structure Step 1: Total valence electrons in SO 4 2- ion First, determine the valence electron available for drawing the Lewis structure of SO 4 2- because the Lewis diagram represents valence electrons around atoms. Draw Lewis dot structure of I3- ion. Boron Family: Borane. Related articles Related Qustion. Hydrogen Isotopes. Condensed Formula. We do this by adding brackets around the ion and showing the charge:. Instantaneous Rate.
A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. The valence electrons are the electrons in the outermost shell.
Speed of Light. Lewis Structures were introduced by Gilbert N. Periodic Trend: Electron Affinity. Introduction to Quantum Mechanics. Transition Metals and Coordination Compounds 0. Law of Conservation of Mass. Formal Charge. Naming Ketones. The Electron Configuration Review. Phase Diagrams. If the ion had been SO 3 2- sulfite instead of sulfate, then the structure would have had a lone pair of electrons on the central atom: The structure of PCl 5 is a good example of a molecule that exceeds the octet: Phosphorus is located in Period 3 3rd row of the periodic table and thus is capable of exceeding an octet. Back to all problems.

Yes, all is logical