Silver molar mass
Wiki User. In terms of metric conversion, silver molar mass of salt is equal to 0. In terms of molar conversion, mg of salt is equal to approximately 0.
The silver molecule consists of 1 Hydrogen atom s - a total of 2 atom s. The molecular weight of silver is determined by the sum of the atomic weights of each constituent element multiplied by the number of atoms, which is calculated to be:. Molecular masses are calculated from the standard atomic weights of each nuclide, while molar masses are calculated from the atomic mass of each element. The atomic mass takes into account the isotopic distribution of the element in a given sample. Our Deep Data encompasses property data, spectral data, quantum chemical data, and molecular descriptor data for a wide range of chemical compounds. It features more than 2, high-quality datasets per single chemical compound, totaling over 8 billion datasets for 4.
Silver molar mass
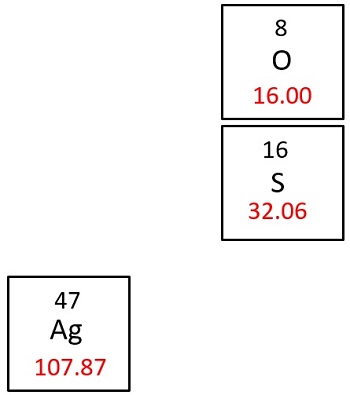
Molar mass of Silver Ag is Then, lookup atomic weights for each element in periodic table : Ag: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:. First, compute the number of each atom in Ag: Ag: 1 Then, lookup atomic weights for each element in periodic table : Ag: Computing molar mass molar weight To calculate molar mass of a chemical compound enter its formula and click 'Compute'. In chemical formula you may use: Any chemical element. Common compound names. Molar mass calculator also displays common compound name, Hill formula, elemental composition, mass percent composition, atomic percent compositions and allows to convert from weight to number of moles and vice versa. Computing molecular weight molecular mass To calculate molecular weight of a chemical compound enter it's formula, specify its isotope mass number after each element in square brackets. Examples of molecular weight computations: C[14]O[16]2 , S[34]O[16]2. Definitions Molecular mass molecular weight is the mass of one molecule of a substance and is expressed in the unified atomic mass units u.
Periodic table.
.
Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. The chemical symbol for Hydrogen is H. With a standard atomic weight of circa 1. Helium is a chemical element with atomic number 2 which means there are 2 protons and 2 electrons in the atomic structure. The chemical symbol for Helium is He. It is a colorless, odorless, tasteless, non-toxic, inert, monatomic gas, the first in the noble gas group in the periodic table. Its boiling point is the lowest among all the elements. Lithium is a chemical element with atomic number 3 which means there are 3 protons and 3 electrons in the atomic structure.
Silver molar mass
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom or group of atoms in the formula by the formula weight and multiplying by Using the chemical formula of the compound and the periodic table of elements, we can add up the atomic weights and calculate molecular weight of the substance. Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. These relative weights computed from the chemical equation are sometimes called equation weights. A common request on this site is to convert grams to moles. To complete this calculation, you have to know what substance you are trying to convert.
Nombre de peliculas para adultos
The molecular weight of silver is determined by the sum of the atomic weights of each constituent element multiplied by the number of atoms, which is calculated to be:. Why are different masses of each element required to react with the same amount of hydrogen? Computing molar mass molar weight To calculate molar mass of a chemical compound enter its formula and click 'Compute'. CO 2 has one carbon atom and two oxygen atoms. Why cant molar mass be used as a conversion factor? Below are some application examples that may interest you:. Find more answers Ask your question. Compound made from Continue Learning about Chemistry. Study now See answer 1. Find atomic masses: look up the atomic masses of each element present in the compound. Related: Molecular weights of amino acids. Home Molecular Weight This Compound. Invalid email address. Computing molecular weight molecular mass To calculate molecular weight of a chemical compound enter it's formula, specify its isotope mass number after each element in square brackets.
A soft, white, lustrous transition metal , it exhibits the highest electrical conductivity , thermal conductivity , and reflectivity of any metal.
What is the molar mass of silver? Common compound names. In terms of molar conversion, mg of salt is equal to approximately 0. Molar mass of Silver Ag is The molecular weight of silver is determined by the sum of the atomic weights of each constituent element multiplied by the number of atoms, which is calculated to be:. Related: Molecular weights of amino acids. Is molar mass the conversion factor in stoichiometry? Molecular Weight Description The silver molecule consists of 1 Hydrogen atom s - a total of 2 atom s. The atomic mass is usually found on the periodic table and is given in atomic mass units amu. Then, lookup atomic weights for each element in periodic table : Ag: Molar mass of Silver Ag is Unit converters. Study now See answer 1.

In my opinion you are mistaken. I suggest it to discuss. Write to me in PM.