Shape of xef2
The hybridization of XeF2 Xenon Difluoride is an sp 3 d type. Here we will try to understand all shape of xef2 steps involved and how to determine this type of hybridization. In the hybridization of xenon difluoride, Xenon Xe is the central atom.
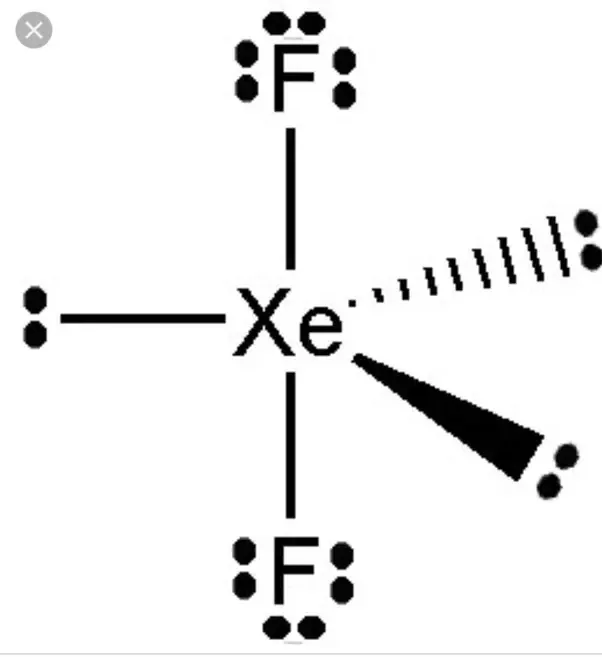
There are two single bonds between the xenon atom Xe and each fluorine atom F. There are three lone pairs of electrons on the xenon atom Xe and on each of the two fluorine atoms F. The XeF2 Lewis structure is shown below:. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. Comparing the electronegativity values of xenon Xe and fluorine F , the xenon atom is less electronegative. Therefore, the xenon atom Xe is the central atom and the fluorine atom F is the external atom. In the case of the XeF2 molecule, the total number of electron pairs is
Shape of xef2
Let us learn about the molecule XeF2, its molecular geometry and bond examples, and XeF2 Lewis structure. The chemical compound Xenon Difluoride is abbreviated as XeF 2. XeF 2 is the most stable of the three chemicals. It is white in colour. Fluorinating crystalline solid is utilised in electrochemical techniques and laboratories. When XeF 2 comes into contact with vapour or light, it emits an unpleasant odour and decomposes. XeF 2 molecular geometry is an important and interesting topic. XeF 2 has a linear molecular geometry. Molecule Xe has eight valence electrons, while fluorine has seven, totalling 22 valence electrons. This indicates that both fluorines must be bound to the Xe molecule, resulting in three unshared pairs and two bonded pairs on the Xe molecule.
Molecules are generated through the formation of certain bonds between atoms that are based on their strength.
.
XeF2 lewis structure is the abbreviation of xenon difluoride. It is one of those rare compounds which involve noble gases despite their strong stability. XeF2 lewis structure and its properties are illustrated in this article. XeF2 lewis structure involves 1 atom of xenon and 2 atoms of fluorine. Xenon has 8 valence electrons and fluorine has 7 valence electrons. So to form a reliable lewis structure xenon will share its 2 electrons with fluorine forming a single covalent Xe-F bond.
Shape of xef2
XeF2 is a covalent inorganic halide formed by the inert gas xenon and the halogen fluorine. This is an active solvent and is found to be soluble in different fluorides like HF and bromine pentafluoride. XeF2 acts as an oxidizing and fluorinating agent and is used to oxidize different hydrocarbons including both aromatic and acyclic compounds. Not only this, but this fluoride compound can also be used to etch silicon to form silicon tetrafluoride SiF4 without any external energy application. If you are thinking about what XeF2 looks like, it appears as a colorless-to-white crystalline solid with a density of around 4.
Tiempo espera garita
Two-hybrid orbitals are used in the formation of F-Xe-F sigma bonds by overlapping the two half-filled 2pz atomic orbitals of fluorine. Allotment of Examination Centre. Step 4 Stability of structure In step 3, the external fluorine atoms form an octet so they are stable, and xenon can form an extended octet that can hold more than 8 electrons and is therefore surrounded by 3 lone pairs of electrons. XeF2 molecular geometry is linear. Here we will try to understand all the steps involved and how to determine this type of hybridization. Homogeneous and heterogeneous molecules are formed when two or more atoms react and combine. An effective anti-wrinkle substance: Acetyl octapeptide Get all the important information related to the JEE Exam including the process of application, important calendar dates, eligibility criteria, exam centers etc. The electrons that participate in bond formation and those that do not are referred to as valence electrons collectively. This is a shift from 2D to 3D structural representation, which allows us to see how a molecule stays in a bonding state in real life. The Lewis structure only shows valence electrons. JEE Main Highlights.
Xenon difluoride is a powerful fluorinating agent with the chemical formula XeF 2 , and one of the most stable xenon compounds. Like most covalent inorganic fluorides it is moisture-sensitive.
Reserved Seats. There are three lone pairs of electrons and two pairs of bond electrons. To create a near-stable composite, this idea minimises the like charge repulsion between negative electron clouds around atomic nuclei. It acquires such shape as the lone pairs present around the central atom tend to take up equatorial positions. Answer: The 4d sublevel will be accessible to xenon with valence electrons at the 4th energy level, allowing for mor JEE Main Highlights. The noble gas xenon difluoride is a hypervalent halogen compound with an octet rule exception and no net dipole moment. Download Now. Is XeF2 a polar or non-polar molecule? Dec 20, XeF2 is a non-polar molecule because the fluorine molecules on either side of the central atom do not have dipole moments and therefore have no polarity. Understanding the geometry of a specific molecule necessitates hybridisation. Mar 16, What is tolnaftate ointment used for? Here we will try to understand all the steps involved and how to determine this type of hybridization.

0 thoughts on “Shape of xef2”