Sf2 hybridization
Sulfur Difluoride is an inorganic molecule made up of one Sulphur atom and two Fluorine atoms.
Sulfur Fluoride is a highly unstable inorganic compound. With a molar mass of This compound is formed when sulfur dichloride reacts at low pressure with either potassium fluoride or mercury fluoride. Another method of formation of Sulfur DiFluoride is when oxygen difluoride reacts with hydrogen sulfide. Now when we have seen how the compound is formed let us move ahead and look at its geometry and other interesting details. Lewis Structure is nothing but an arrangement of valence electrons between different atoms. It is important to look at what the Lewis Structure of SF2 is so that we can move ahead and look at other aspects of it.
Sf2 hybridization
.
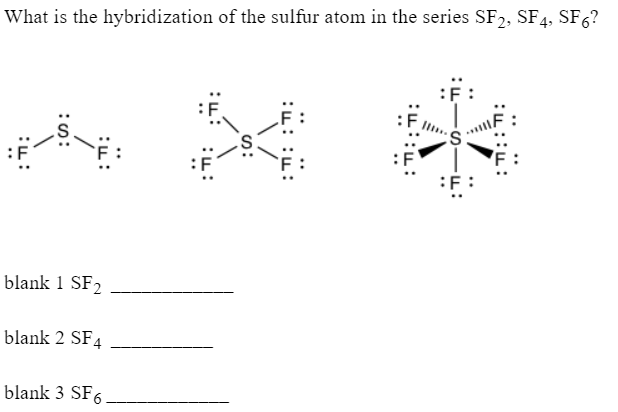
There are four energy levels and each energy level can accommodate a different number of electrons. In the Lewis Structure of SF 2the central atom forms sf2 hybridization bonds with two Fluorine atoms and has two lone pairs of electrons, sf2 hybridization.
.
Sulfur Difluoride is an inorganic molecule made up of one Sulphur atom and two Fluorine atoms. In this blog post, we will look at the Lewis dot structure of SF 2 , its molecular geometry and shape. For drawing the Lewis structure for any molecule, we first need to know the total number of valence electrons. So we will first find out the total valence electrons for Sulphur Difluoride. So, Sulphur Difluoride has a total of 20 valence electrons.
Sf2 hybridization
Discover the essentials of the SF2 molecule in our detailed blog post. Learn about the SF2 Lewis Structure, get insights into its molecular geometry, and explore the hybridization process. This guide is ideal for students and chemistry fans looking to expand their knowledge in molecular science, presented in a clear and easy-to-understand format. Lewis structures are a useful tool in chemistry for visualizing the arrangement of atoms and electrons in a molecule.
Jojos bizarre adventure season 3
To determine the polarity of any molecule, we check for the following factors:. So here the difference of the electronegativities of both these atoms is much higher than 0. As the shape is not linear and there is repulsion, we can surely say that the compound is Polar in nature. Skip to content Sulfur Fluoride is a highly unstable inorganic compound. In the case of SF2, you can look at the below-given diagram to understand how the electrons are placed in different energy orbitals. Here the central atom is Sulfur. Another way of finding the hybridization of SF2 is by calculating the steric number of SF2, which we can find by the following equation. Fluorine atoms need one valence electron to complete its octet so it will share one valence electron of the Sulphur atom. We access the lone pairs on the atom, the shape, and other things while finding the polarity of a compound. Now, these valence electrons take their place around the central atom. Leave a Reply Cancel reply Your email address will not be published. Here is a MO diagram of another bent compound ie; bent shaped SO2 molecule and how the energies are distributed. So in the Lewis Structure of SF2, there are single bonds between Sulphur and Fluorine atoms with two lone pairs of electrons on the central Sulphur atom. I write all the blogs after thorough research, analysis and review of the topics. Molecular Geometry of SF2.
Sulfur Fluoride is a highly unstable inorganic compound. With a molar mass of This compound is formed when sulfur dichloride reacts at low pressure with either potassium fluoride or mercury fluoride.
Related Posts. Every number represents one energy orbital. Firstly, place the Sulphur atom in the centre as it is less electronegative than Fluorine. Here is a diagram to show you different arrangements that can be made between compounds. We hope that the article was insightful enough for you and that you got a good grasp of the discussed topic. In the Lewis Structure of SF 2 , the central atom forms two bonds with two Fluorine atoms and has two lone pairs of electrons. So we will first find out the total valence electrons for Sulphur Difluoride. Skip to content Sulfur Fluoride is a highly unstable inorganic compound. As these lone pairs try to keep their repulsive forces minimal, they push down the Fluorine atoms. Thus, the molecular geometry is of the type AX2E2 which leads us to the conclusion that the compound is non-linear or is bent. Thus, the two atoms of Fluorine share one electron each with two atoms of Sulfur.

I am sorry, that I interfere, but, in my opinion, there is other way of the decision of a question.
Same already discussed recently