Series limit of balmer series
The series limit wavelength of the Lyman series for the hydrogen atom is given by. Balmer series of hydrogen atom lies in.
Balmer series of hydrogen atom lies in. The series limit for Balmer series of H-spectra is. The series limit wavelength of the Balmer series for the hydrogen atom is. The energy of the highest energy photon of Balmer series of hydrogen atom is close to. The wavelength of the third line of the Balmer series for a hydrogen atom is -. Write an empirical relation for the Balmer series of hydrogen atom.
Series limit of balmer series
A sequence of absorption or emission lines in the visible part of the spectrum, due to hydrogen; also known as Balmer lines. Balmer absorption lines are caused by jumps of electrons from the second energy level to higher levels, and emission lines when the electrons drop back to the second energy level. They are named after the Swiss mathematician Johann Jakob Balmer — See also hydrogen spectrum. From: Balmer series in A Dictionary of Astronomy ». Subjects: Science and technology — Astronomy and Cosmology. View all related items in Oxford Reference ». Search for: 'Balmer series' in Oxford Reference ». All Rights Reserved. Under the terms of the licence agreement, an individual user may print out a PDF of a single entry from a reference work in OR for personal use for details see Privacy Policy and Legal Notice. Personal Profile. Oxford Reference. Publications Pages Publications Pages. Recently viewed 0 Save Search.
The series continues with an infinite number of lines whose wavelengths asymptotically approach the limit of The following figure indicates the energy levels of a certain atom. What will be the ratio of de - Broglie wavelengths of proton and alpha
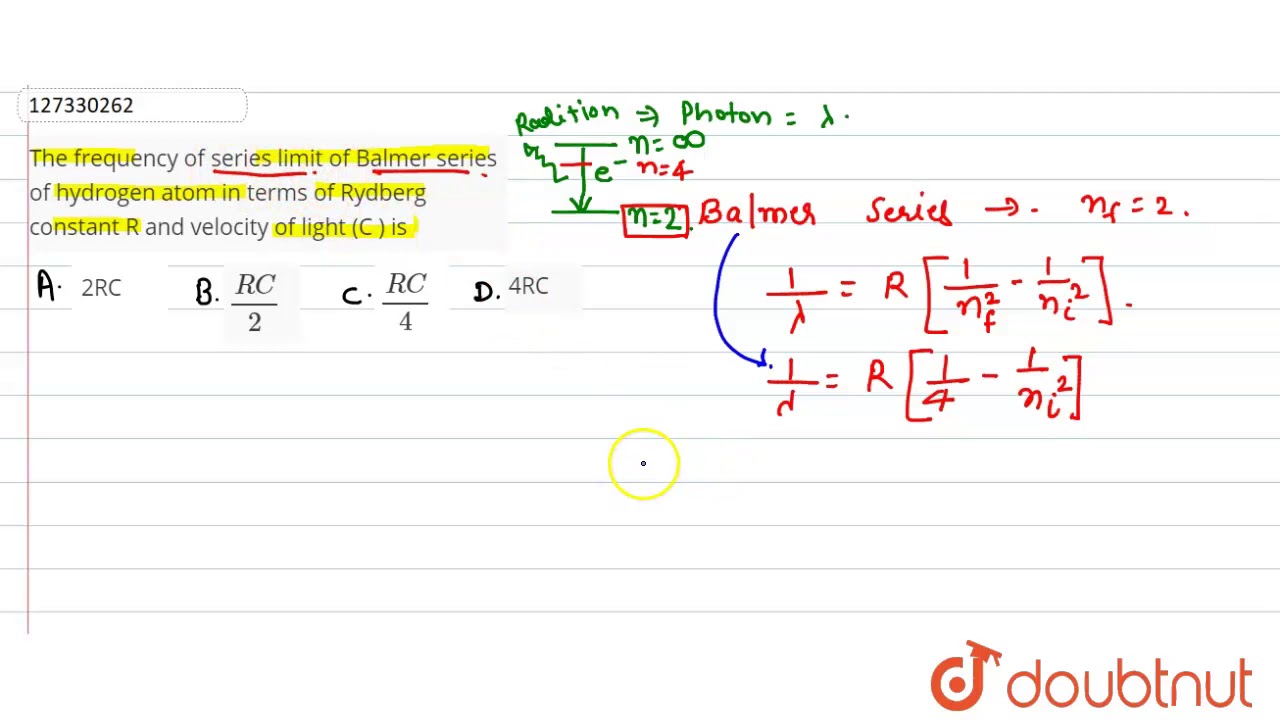
This action cannot be undone. This will permanently delete All Practiced Questions. The frequency of the series limit of the Balmer series of hydrogen atoms in terms of Rydberg constant R and velocity of light C is:. Orbital acceleration of electrons is: 1. The shortest wavelength is given by:. The distance of closest approach is of the order.
This orbit is called the ground state. These higher orbits are called excited states. When electrons start revolving in the excited state the atom becomes unstable. This radiation is emitted in the form of spectral lines. The Expression for the Wavelength of a line in the Hydrogen Spectrum:. We get different series of spectral lines due to the transition of the electron from different outer orbits to fixed inner orbit. Energy Level Diagram for Hydrogen Atom:. Energy level diagrams indicate us the different series of lines observed in a spectrum of the hydrogen atom. The horizontal lines of the diagram indicate different energy levels. The vertical lines indicate the transition of an electron from a higher energy level to a lower energy level.
Series limit of balmer series
The first person to realize that white light was made up of the colors of the rainbow was Isaac Newton, who in passed sunlight through a narrow slit, then a prism, to project the colored spectrum on to a wall. This effect had been noticed previously, of course, not least in the sky, but previous attempts to explain it, by Descartes and others, had suggested that the white light became colored when it was refracted, the color depending on the angle of refraction. Newton clarified the situation by using a second prism to reconstitute the white light, making much more plausible the idea that the white light was composed of the separate colors. He then took a monochromatic component from the spectrum generated by one prism and passed it through a second prism, establishing that no further colors were generated. That is, light of a single color did not change color on refraction. He concluded that white light was made up of all the colors of the rainbow, and that on passing through a prism, these different colors were refracted through slightly different angles, thus separating them into the observed spectrum.
Google drive dan whatsapp mesajlarını okuma
Read Edit View history. Toggle limited content width. Question Type. NEET Questions. Subtopic: Various Atomic Models. In true-colour pictures, these nebula have a reddish-pink colour from the combination of visible Balmer lines that hydrogen emits. The energy of the highest energy photon of Balmer series of hydrogen atom is close to. Write an empirical relation for the Balmer series of hydrogen atom. In stars , the Balmer lines are usually seen in absorption, and they are "strongest" in stars with a surface temperature of about 10, kelvins spectral type A. View Solution. Subjects: Science and technology — Astronomy and Cosmology. Georgia State University. This action cannot be undone. His number also proved to be the limit of the series.
In an amazing demonstration of mathematical insight, in Balmer came up with a simple formula for predicting the wavelength of any of the lines in atomic hydrogen in what we now know as the Balmer series. Three years later, Rydberg generalized this so that it was possible to determine the wavelengths of any of the lines in the hydrogen emission spectrum.
The nucleus of a hydrogen atom is In the following reaction. The nucleus of a hydrogen atom is. If p is the moment of Clear Question Attempted. Generally the approximate limits of visible spectrum are Your current browser may not support copying via this button. Facebook LinkedIn Twitter. Contents move to sidebar hide. The frequency of the series limit of the Balmer series of hydrogen atoms in terms of Rydberg constant R and velocity of light C is: 1. The frequnecy of visible light is of the order of. The distance of closest approach is of the order. The series limit wavelength of the Lyman series for the hydrogen atom is given by.

I apologise, but, in my opinion, you are not right. I am assured. I can prove it. Write to me in PM, we will communicate.
I think, that you are not right. I am assured. I can prove it. Write to me in PM.