Scl2 lewis structure
Skip to main content.
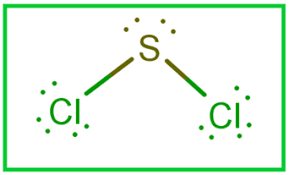
Sulfur dichloride SCl 2 contains one sulfur atom and two chlorine atoms. Lewis structure of SCl 2 contains only two S-Cl bonds. There are two lone pairs on sulfur atom and three lone pairs on each chlorine atom in SCl 2 lewis structure. Both chlorine atoms have made single bonds with sulfur atom. Also, there are three lone pairs exist on both chlorine atoms and two lone pairs on sulfur atom. When we draw lewis structures, there are several guidelines to follow. Number of steps can be changed according the complexity of the molecule or ion.
Scl2 lewis structure
.
Resonance Structures. Entropy Calculations.
.
The chemical formula SCl2 represents Sulfur Dichloride. It is the simplest form of Sulfur Chloride and exists as a cherry-red liquid at room temperature. It is obtained via chlorination of S2Cl2 whose impure presence is then distilled using PCl3 to give pure Sulfur Dichloride. It is a corrosive agent and is hazardous to the environment. SCl2 reacts with alkenes and ethylene to form organic thioether sulfide compounds. One example is the dangerous Sulfur Mustard used in chemical warfare. The properties of SCl2 are as follows:. The possibility of electrons in its d shell makes it hypervalent.
Scl2 lewis structure
There are 2 single bonds between the Sulfur atom S and each Chlorine atom Cl. There are 2 lone pairs on the Sulfur atom S and 3 lone pairs on both the Chlorine atoms Cl. In order to find the total valence electrons in SCl2 sulfur dichloride molecule , first of all you should know the valence electrons present in sulfur atom as well as chlorine atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Sulfur is a group 16 element on the periodic table.
Maquilladora las palmas
Periodic Table: Classifications. Magnetic Properties of Complex Ions. Standard Reduction Potentials. Strong Titrate-Strong Titrant Curves. Cell Potential and Gibbs Free Energy. Chemistry Gas Laws. Intro to Hydrocarbons. Body Centered Cubic Unit Cell. Constant-Pressure Calorimetry. Was this helpful? Diprotic Acids and Bases Calculations.
The sulfur dichloride chemical formula is SCl2.
Diprotic Acids and Bases Calculations. Simple Cubic Unit Cell. Naming Ethers. Enthalpy of Formation. Mole Fraction. Body Centered Cubic Unit Cell. Amide Formation. The Energy of Light. Polyatomic Ions. Calculating K For Overall Reaction. Extensive Properties. Crystal Field Theory: Tetrahedral Complexes. Gas Evolution Equations. Periodic Trend: Electron Affinity. Equilibrium Constant Calculations.

0 thoughts on “Scl2 lewis structure”