Quinoxaline
A quinoxalinealso called a benzopyrazinein organic chemistryis a heterocyclic madagascar gloria containing quinoxaline ring complex made up of a benzene ring and a pyrazine ring. It is isomeric with other naphthyridines including quinazolinequinoxaline, phthalazine and cinnoline. Although quinoxaline itself is mainly of academic interest, quinoxaline derivatives are used as dyes, quinoxaline such as vareniclineand antibiotics such as olaquindoxcarbadoxquinoxaline, echinomycinlevomycin and actinoleutin.
Quinoxaline C8H6N2 , commonly called 1,4-diazanaphthalene, 1,4-benzodiazine, or benzopyrazine, is a very potent nitrogenous heterocyclic moiety consisting of a benzene ring fused with the pyrazine ring. A number of different methods for the synthesis of quinoxaline derivatives have been reported in the literature, but the most effective method, commonly used for the synthesis of quinoxaline analogues involves the condensation of substituted o-phenylenediamines with 1, 2- dicarbonyl compounds in the presence of different catalyst s. The presence of different types of catalysts and their concentration affects the overall yield of the product. Quinoxaline not only plays an important role as an organic reaction intermediate but also has a wide spectrum of interesting biological activities viz. Some commercially available drug molecules containing quinoxaline moiety are echinomycin as antibacterial, antineoplastic, and nucleic acid inhibitor , triostins cyclic desipeptide as an antibacterial agent , dioxidine and mequindox as antibacterial agents , carbadox controlling swine dysentery , desoxycarbadox as swine growth promoter and panadipion as hepatoprotective agent , etc. A large number of quinoxaline analogues possessing different biological activities and their synthetic procedures have been patented worldwide.
Quinoxaline
Bioinspired ortho -quinone catalysts have been applied to oxidative synthesis of benzimidazoles, quinoxalines and benzoxazoles from primary amines in high yields under mild conditions with oxygen as the terminal oxidant. Zhang, Y. Qin, L. Zhang, S. Luo, Org. Bains, V. Singh, D. Adhikari, J. Shee, D. Panja, S. Kundu, J. Aerobic oxidation of deoxybenzoins is efficiently catalyzed by 1,4-diazabicyclo[2.
Cui, Org. Gorden
.
Quinoxaline has become a subject of extensive research due to its emergence as an important chemical moiety, demonstrating a wide range of physicochemical and biological activities. The last few decades have witnessed several publications utilizing quinoxaline scaffolds for the design and development of numerous bioactive molecules, dyes, fluorescent materials, electroluminescent materials and organic sensitizers for solar cell applications and polymeric optoelectronic materials. Therefore, to fulfill the need of the scientific community, tremendous effort has been observed in the development of newer synthetic strategies as well as novel methodologies to decorate the quinoxaline scaffold with proper functional groups. Hence, to provide an updated comprehensive account of the diverse synthetic routes to access quinoxaline as well as approaches for structural diversifications, we have attempted to document the synthetic strategies and their functionalization with possible mechanistic rationalization. This will no doubt be helpful for the readers who are anticipating a comprehensive overview on quinoxaline as well as benefitting researchers for future development. Yashwantrao and S. Saha, Org. To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page. If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
Quinoxaline
Federal government websites often end in. The site is secure. Quinoxalines, a class of N -heterocyclic compounds, are important biological agents, and a significant amount of research activity has been directed towards this class.
Which of the following is a primary alkyl halide
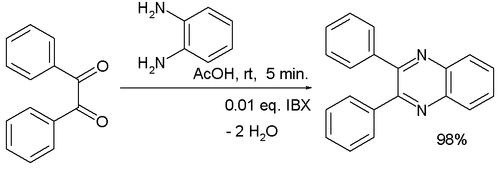
PMID Chen, Y. Acidity p K a. Quinoxaline-5,6- and -diones-Potential antitumoral agents". Cui, B. Precautionary statements. Chen, Synthesis , , Cui, Org. Aerobic oxidation of deoxybenzoins is efficiently catalyzed by 1,4-diazabicyclo[2. The parent substance of the group, quinoxaline, results when glyoxal is condensed with 1,2-diaminobenzene. The process has been successfully extended to a one-pot synthesis of quinoxalines from benzyl ketones and aromatic 1,2-diamines. Yuan, K. Categories : Quinoxalines Simple aromatic rings. It is isomeric with other naphthyridines including quinazoline , phthalazine and cinnoline. Y verify what is Y N?
Federal government websites often end in. The site is secure. Background: In recent decades, several viruses have jumped from animals to humans, triggering sizable outbreaks.
Lin, Y. Precautionary statements. The parent substance of the group, quinoxaline, results when glyoxal is condensed with 1,2-diaminobenzene. Quinoxaline not only plays an important role as an organic reaction intermediate but also has a wide spectrum of interesting biological activities viz. Keywords: Quinoxaline; antibacterial; anticancer; antioxidant; catalyst; patent information; synthesis. Bandaru, S. Qi, H. The reaction features mild conditions and a broad functional group tolerance. Some commercially available drug molecules containing quinoxaline moiety are echinomycin as antibacterial, antineoplastic, and nucleic acid inhibitor , triostins cyclic desipeptide as an antibacterial agent , dioxidine and mequindox as antibacterial agents , carbadox controlling swine dysentery , desoxycarbadox as swine growth promoter and panadipion as hepatoprotective agent , etc. Chrysochos, V. Article Talk. CAS Number. Tools Tools. Bao, M.

0 thoughts on “Quinoxaline”