Polarity of ph3
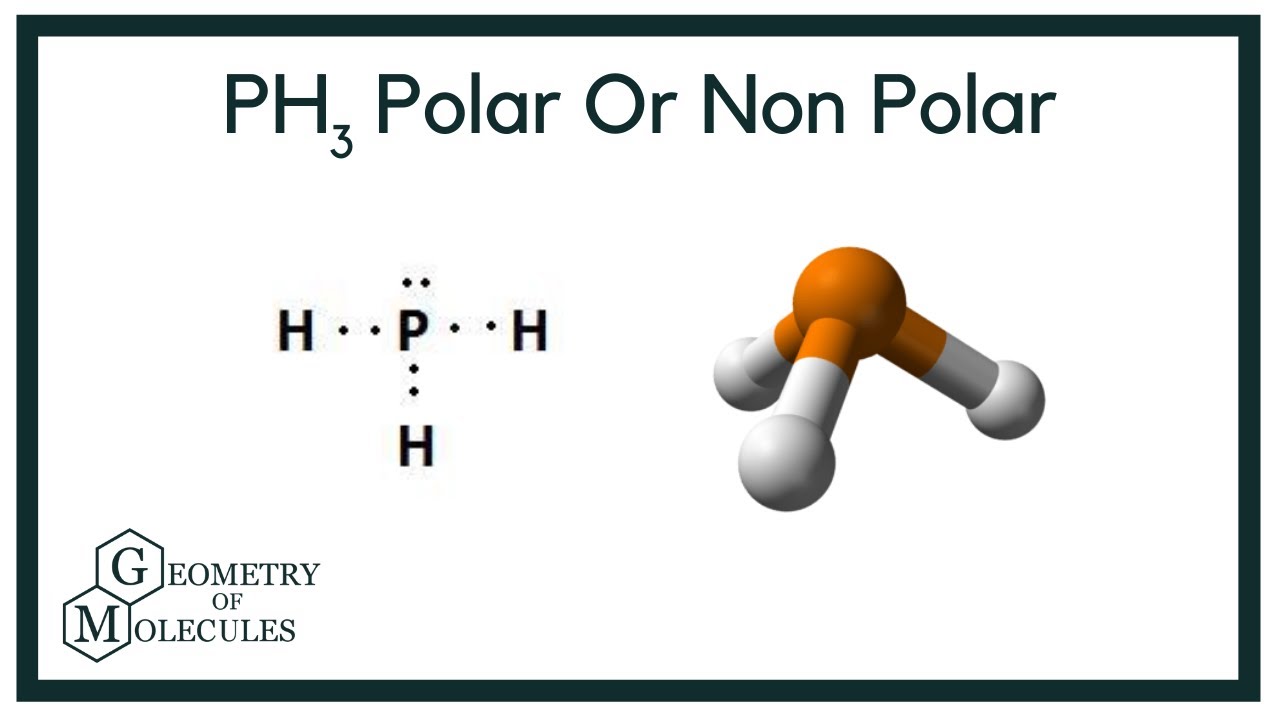
Is PH3 Polar or Nonpolar? Answer: PH3 is polar due to the presence of a lone pair of electrons with electron-electron repulsion causing an overall "bent" structure. This results in a dipole moment throughout the molecule.
Hence, the compound is crushed to the core and gains the ability to heat a talk to a debate. Its pure form is odorless, and other forms have an unpleasant odor like a rotten fish, or garlic, due to the presence of substituted Phosphine and Diphosphane. So, is PH3 Polar or Nonpolar? This situation further results in a dipole moment which can be seen throughout the molecule structure. PH3 Phosphine is also polar because of the rule that all polar molecules must contain polar bonds which are formed due to a difference in electronegativity found between the bonded atoms of the chemical compound.
Polarity of ph3
.
What state is PH3 normally found in? This chart is very important hence, it helps to answer such questions.
.
Whether a bond is nonpolar or polar covalent is determined by a property of the bonding atoms called electronegativity. Electronegativity is a measure of the tendency of an atom to attract electrons or electron density towards itself. It determines how the shared electrons are distributed between the two atoms in a bond. The more strongly an atom attracts the electrons in its bonds, the larger its electronegativity. Electrons in a polar covalent bond are shifted toward the more electronegative atom; thus, the more electronegative atom is the one with the partial negative charge.
Polarity of ph3
Molecules have shapes. There is an abundance of experimental evidence to that effect — from their physical properties to their chemical reactivity. Small molecules — molecules with a single central atom — have shapes that can be easily predicted. It says that electron pairs, being composed of negatively charged particles, repel each other to get as far away from each other as possible. VSEPR makes a distinction between electron group geometry , which expresses how electron groups bonding and nonbonding electron pairs are arranged, and molecular geometry , which expresses how the atoms in a molecule are arranged.
Koda capital
Lewis structure of Phosphine or PH3. A compound becomes Polar when an electronegativity difference is found between the bonded atoms. And as a rule of polarity, any molecule with a positive along with a negative region will be considered polar. It is generally classed as a pnictogen hydride in chemistry. So, is PH3 Polar or Nonpolar? Save my name, email, and website in this browser for the next time I comment. For deep knowledge, you must read an article on the lewis structure of PH3. How is Phosphine PH3 formed and why it is Insoluble? PH3 Ball and Stick Model. Phosphine structurally looks like ammonia NH3 , but on the contrary PH3 acts as a poor solvent than ammonia and hence is much less soluble in water, to the extent that it is called insoluble with Phosphine is the best example of a polar molecule having non-polar bonds.
The compound Phosphorous Trihydride PH3 , also known as phosphine consists of phosphorus and hydrogen atoms.
For deep knowledge, you must read an article on the lewis structure of PH3. What state is PH3 normally found in? So, the molecular geometry for ph3 is Trigonal Pyramidal. In the formation of the chemical compound phosphine, pure p orbitals take part in bonding and avoid getting hybridized. Newer Post Older Post Home. For more understanding, check out the article for the polarity of NCl3. Hence, Phosphine is a Polar molecule. The PH3 Lewis structure has 8 valence electrons. In the PH3 molecule, the Phosphorus atom has 5 valance electrons. It is generally classed as a pnictogen hydride in chemistry.

I confirm. So happens. We can communicate on this theme. Here or in PM.