Pkb meaning in chemistry
For strong acids, i. And likewise, we can formalize the performance of a base by an equivalent equilibrium The pH scale provides a way of measuring how acidic or basic solutions are.
It is used to determine the strength of a base or alkaline solution. As with the acid dissociation constant , pK a , the base dissociation constant calculation is an approximation that is only accurate in dilute solutions. Kb can be found using the following formula:. The base dissociation constant is related to the acid dissociation constant, so if you know one, you can find the other value. At the same ionic strength and temperatures:. Find the value of the base dissociation constant K b and pK b for a 0. First calculate the hydrogen and hydroxide ion concentrations in the solution to get values to plug into the formula.
Pkb meaning in chemistry
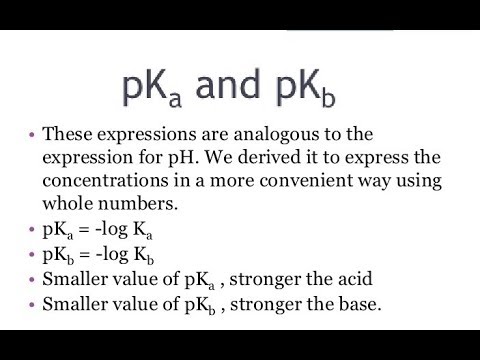
It is used to measure basic strength. The lesser the pKb is, the more potent the base will be. It is equivalent to the negative logarithm of base dissociation constant, Kb. It tells us about how much a base dissociates in an aqueous solution. Kb is used in distinguishing a strong base from a weak base. More the value of Kb more would be its dissociation. A strong base will dissociate into its constituent ions quickly. Thus, we can say that a strong base has a larger Kb. The dissociation constant of a strong base is as high as 10 2 , while a weak base has as low as 10 , which is quite challenging to remember. So to ease that, pKb came into existence. Putting the base dissociation constant value in the equation, we get -2 pKb for a strong base and 10 pKb for a weak base. Putting the base dissociation constant value in the equation, we get -2 pKb for a strong base and 10 pKb for a weak base, which is easy to remember.
The magnitude of the equilibrium constant for an ionization reaction can be used to determine the relative strengths of acids and bases. How does pKa change with environment?
There are related scales in chemistry used to measure how acidic or basic a solution is and the strength of acids and bases. Although the pH scale is most familiar, pKa , Ka , pKb , and Kb are common calculations that offer insight into acid-base reactions. Here's an explanation of the terms and how they differ from each other. Whenever you see a "p" in front of a value, like pH, pKa , and pKb, it means you're dealing with a -log of the value following the "p". For example, pKa is the -log of Ka.
There are related scales in chemistry used to measure how acidic or basic a solution is and the strength of acids and bases. Although the pH scale is most familiar, pKa , Ka , pKb , and Kb are common calculations that offer insight into acid-base reactions. Here's an explanation of the terms and how they differ from each other. Whenever you see a "p" in front of a value, like pH, pKa , and pKb, it means you're dealing with a -log of the value following the "p". For example, pKa is the -log of Ka. Because of the way the log function works, a smaller pKa means a larger Ka. If you know pH, you can calculate pOH.
Pkb meaning in chemistry
The pH scale is the most familiar measure of acidity and basicity, but pKa, pKb, Ka, and Kb are better for predicting acid and base strength and their reactions. Here are definitions of each term, simple formulas used to calculate them, and an explanation of how they differ from one another. So, pH is the negative log of hydrogen ion concentration, while pKa is the negative log of the Ka value.
Car hire fort lauderdale cruise terminal
How do you find the pH? It is used to determine the strength of a base or alkaline solution. The pKa gives the same information, just in a different way. Definition and Examples of Acid-Base Indicator. Use limited data to select content. The base dissociation constant is a measure of how completely a base dissociates into its component ions in water. Please explain , thanks. List of Partners vendors. And likewise, we can formalize the performance of a base by an equivalent equilibrium How does a pKa value relate to pH? We convert these exponential numbers into a normal range by taking their negative logarithm. Why does toluene have a lower pka than benzene? Henderson Hasselbalch Equation Definition. Another important point is pI.
A measure of the strength of an acid on a logarithmic scale.
In contrast, a small Ka value means only a small amount of acid dissociates, indicating a weak acid. How could you chemically identify "phthalic acid" , "oxalic acid" , and "oxalic acid dihydrate"? Butyric acid is responsible for the foul smell of rancid butter. Synthetic Fibres. How does pKa relate to basicity? Of the following which has the lowest pKa value? What is the structure of glycine at pH 5? Anne Marie Helmenstine, Ph. Now, you have the necessary information to solve for the base dissociation constant:. If given a pH of 2. What are Ka and Kb in acids and bases? What is the chemical importance of pKb? There are related scales in chemistry used to measure how acidic or basic a solution is and the strength of acids and bases. Use limited data to select content.

I can recommend to visit to you a site, with a large quantity of articles on a theme interesting you.