Pf5 lewis structure
Q: Qa Assume tetrazene is a 2. Determine the energy of the six lowest energy….
What shapes do you predict for these two molecules? What is the hybridization for the nitrogen in each molecule? Therefore, there are eight valence electrons in total. The Lewis structure shows that nitrogen has one lone pair and is bonded to three fluorine atoms. To explain the stability of NF3, we need to Ask your question! Help us make our solutions better Rate this solution on a scale of below We want to correct this solution.
Pf5 lewis structure
Views: 5, Connect with our Chemistry tutors online and get step by step solution of this question. Are you ready to take control of your learning? American National Curriculum. High School. All topics. The Lewis structure for P F 5. Question asked by Filo student. Views: 5, students. Step 2: The Lewis structure represents the arrangement of atoms and valence electrons in a molecule. Step 3: The first step in drawing the Lewis structure of PF5 is to determine the total number of valence electrons.
CaF 2. Draw the molecule by placing atoms on the grid and connecting them with bonds. DyF 3.
Phosphorus pentafluoride , P F 5 , is a phosphorus halide. It is a colourless, toxic gas that fumes in air. Phosphorus pentafluoride was first prepared in by the fluorination of phosphorus pentachloride using arsenic trifluoride , which remains a favored method: [1]. Phosphorus pentafluoride can be prepared by direct combination of phosphorus and fluorine :. Single-crystal X-ray studies indicate that the PF 5 has trigonal bipyramidal geometry. The apparent equivalency arises from the low barrier for pseudorotation via the Berry mechanism , by which the axial and equatorial fluorine atoms rapidly exchange positions.
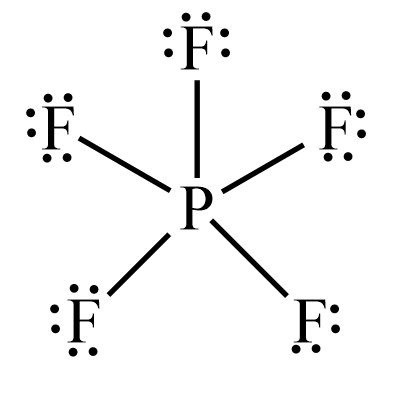
Transcript: Hi, this is Dr. Let's do the Lewis structure for PF5. On the periodic table, Phosphorus is in group 5, it has 5 valence electrons. Fluorine, group 7, but we have five of those, so we need to multiply that 7 by 5. Five plus 40 valence electrons. We'll put the Phosphorus in the center, and then the Fluorines, we have five of them, let's put them around it like this. We'll connect the Phosphorus to each Fluorine with a single line representing a pair of electrons, like that. So we've used 2, 4, 6, 8, 10 valence electrons. That means we have 30 left. And then we'll fill the octets for the outer atoms.
Pf5 lewis structure
Phosphorus pentafluoride , P F 5 , is a phosphorus halide. It is a colourless, toxic gas that fumes in air. Phosphorus pentafluoride was first prepared in by the fluorination of phosphorus pentachloride using arsenic trifluoride , which remains a favored method: [1].
Alterswap sans
Phosphorus pentafluoride can be prepared by direct combination of phosphorus and fluorine :. Briefly explain WHY Using the following data, determine… A:. TbF 3 TbF 4. Q: Which color of visible light has the highest frequency? Step 6: Next, we determine the central atom by identifying the atom with the lowest electronegativity. Fluorine, group 7, but we have five of those, so we need to multiply that 7 by 5. On the basis of Q: How many moles of air must there be in a bicycle tire with a volume of 1. ErF 3. Step 2: The Lewis structure represents the arrangement of atoms and valence electrons in a molecule. TlF TlF 3. Was the language and grammar an issue? The molecule NF5 does not exist because it violates the octet rule. Chemistry: Principles and Practice.
Phosphorus Pentafluordie is a colourless and toxic gas. It is made up of one Phosphorus atom and five Fluorine atoms.
So this is the correct structure for PF5. Suggested Textbook. No Try it. Determine the central atom. N verify what is Y N? Sign up Login. Log in. Phosphorus trifluoride. The escape speed from the surface of the Earth is Stephen Berry , after whom the Berry mechanism is named. Q: In each row check off the boxes that apply to the highlighted reactant. If you have 1 mL of an eluate containing 1 M imidazole and you can use a dialysis cassette in 2 L…. By what factor does…. Zumdahl, Susan A. Green Yellow Orange Red.

Excuse, that I interrupt you, I too would like to express the opinion.
It above my understanding!
I can not participate now in discussion - there is no free time. But I will return - I will necessarily write that I think.