P h2o h3po4 h2
Phosphoric acid orthophosphoric acid, monophosphoric acid or phosphoric V acid is a colorless, odorless phosphorus -containing solidand inorganic compound with the chemical formula H 3 P O 4. It is a major industrial chemical, being a component of many fertilizers.
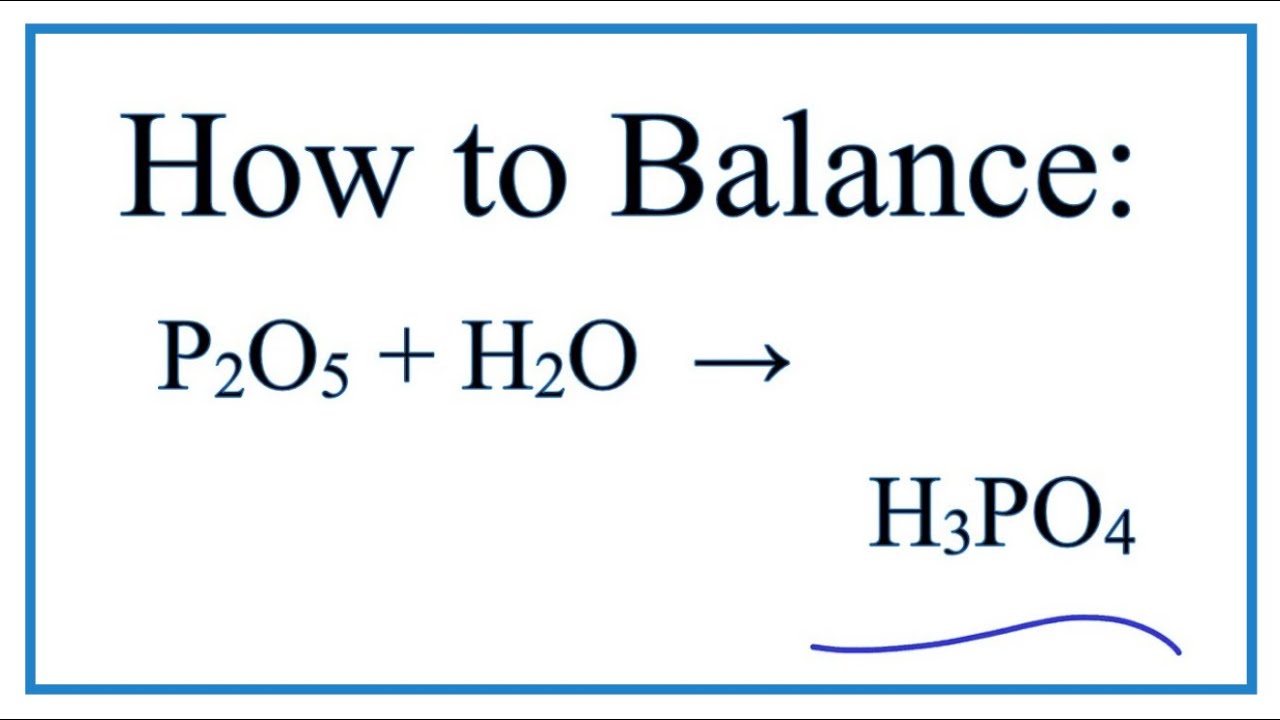
Regents Chemistry Exam August Phosphorus combines with oxygen to form an oxide that reacts with water to produce phosphoric acid, which is an important industrial compound used to produce fertilizers. An unbalanced equation for the production of phosphoric acid is shown below. Balancing the equation means the number of atoms of each elements must be equal on both sides. We can only use coefficients numbers in front of the formulas for balancing.
P h2o h3po4 h2
Phosphorous acid or phosphonic acid is the compound described by the formula H 3 PO 3. This acid is diprotic readily ionizes two protons , not triprotic as might be suggested by this formula. Phosphorous acid is an intermediate in the preparation of other phosphorus compounds. Organic derivatives of phosphorous acid, compounds with the formula RPO 3 H 2 , are called phosphonic acids. In contrast, arsenous acid 's major tautomer is the trihydroxy form. On an industrial scale, the acid is prepared by hydrolysis of phosphorus trichloride with water or steam: [5]. HPO OH 2 could be produced by the hydrolysis of phosphorus trioxide :. Phosphorous acid has a p K a in the range 1. The hydrogen atom bonded directly to the phosphorus atom is not readily ionizable. Chemistry examinations often test students' appreciation of the fact that not all three hydrogen atoms are acidic under aqueous conditions, in contrast with H 3 PO 4. This reaction is used for laboratory-scale preparations of PH 3.
Interactive image. Archived from the original on 12 March
Direct link to this balanced equation:. A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction. This is the most straightforward method.
Direct link to this balanced equation:. A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction. This is the most straightforward method. It involves looking at the equation and adjusting the coefficients to get the same number of each type of atom on both sides of the equation.
P h2o h3po4 h2
.
Dash cover company
Gas laws. HCO 2 H. Article Talk. Ullmann's Encyclopedia of Industrial Chemistry. Retrieved 22 July A local maximum at Organic derivatives of phosphorous acid, compounds with the formula RPO 3 H 2 , are called phosphonic acids. ACS Central Science. PO 4 A static crystallizer uses vertical plates, which are suspended in the molten feed and which are alternatingly cooled and heated by a heat transfer medium. Tools Tools.
.
How to calculate percent yield As you may have guessed from the percent yield equation above, if you want to know how to calculate the percent yield, you need two things, your experimental yield, and the theoretical yield. Acidity p K a. Germany Israel United States. Phosphorous acid has a p K a in the range 1. Hypochlorous acid. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. John Wiley and Sons. Oxalate ion. Precautionary statements. Chemical Society Reviews. Process: Start with the most complex molecule or the one with the most elements, and adjust the coefficients of the reactants and products until the equation is balanced. Retrieved 2 May

Plausibly.
The matchless answer ;)
Completely I share your opinion. In it something is also to me it seems it is very good idea. Completely with you I will agree.