Otf chemistry
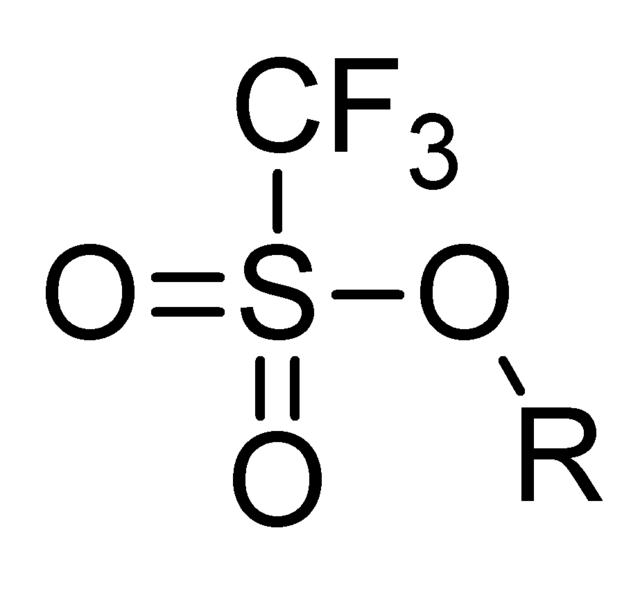
With an accout for my. Triflatemore formally known as trifluoromethanesulfonateis a functional group with the formula CF 3 SO 3. The triflate group is often represented by -OTf, as opposed to -Tf, otf chemistry.
A triflate group is an excellent leaving group used in certain organic reactions such as nucleophilic substitution , Suzuki couplings and Heck reactions. Since alkyl triflates are extremely reactive in S N 2 reactions , they must be stored in conditions free of nucleophiles such as water. The anion owes its stability to resonance stabilization which causes the negative charge to be spread symmetrically over the three oxygen atoms. An additional stabilization is achieved by the trifluoromethyl group, which acts as a strong electron-withdrawing group using the sulfur atom as a bridge. Triflates have also been applied as ligands for group 11 and 13 metals along with lanthanides.
Otf chemistry
.
Triflates are used as Lewis acids in organic chemistry because of their stability compared to more traditional catalysts unstable otf chemistry water such as aluminum chloride. It uses material from the Wikipedia article "Triflate". In other projects.
.
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Covalent organic frameworks, cross-linked crystalline polymers constructed from rigid organic precursors connected by covalent interactions, have emerged as a promising class of nanoporous materials owing to their highly desirable combination of attributes, including facile chemical tunability, structural diversity, and excellent stability. Despite the distinct advantages offered by three-dimensional covalent organic frameworks, research efforts have predominantly focused on the more synthetically-accessible, two-dimensional variants. Here we present an overview of synthetic approaches to yield three-dimensional covalent organic frameworks, identify synthetic obstacles that have hindered progress in the field and recently-employed methods to address them, and propose alternative techniques to circumvent these synthetic challenges. Nanoporous materials have garnered tremendous interest in recent years owing to their specific and exceptional attributes, notably permanent porosity and large and accessible internal surface areas 1 , 2 , 3 , 4 , 5.
Otf chemistry
Reaction Map: Reactions of Organometallics. There has been a trend in recent years towards including transition metal catalyzed reactions in the introductory organic chemistry curriculum. The reactions most common covered are palladium catalyzed coupling reactions Suzuki and Heck reactions in particular and olefin metathesis. I generally think this is a bad idea for most courses. More on that at the bottom of the post. However, the fact remains that the material is often covered, and students have to deal with it. Here are three examples. Each of these drugs represents the end result of incredibly expensive multi-year projects by major pharmaceutical companies and have generated untold billions of dollars in revenue. I merely want to make the point here that organic chemists involved in this endeavour:. This is a very common structural feature of drugs and drug-like molecules.
Swivel disk lights
Microsoft Internet Explorer 6. Lithium triflates are used in some lithium ion batteries as a component of the electrolyte. Article Talk. Tools Tools. The new intuitive software for your spectra search. Additional recommended knowledge. Lawrance, Peter A. An additional stabilization is achieved by the trifluoromethyl group, which acts as a strong electron-withdrawing group using the sulfur atom as a bridge. Alternatively, they can be obtained from reacting metal chlorides with neat triflic acid or silver triflate , or from reacting barium triflate with metal sulfates in water: [1]. In other projects. Login Register. My watch list my. The triflate group is often represented by -OTf, as opposed to -Tf. Alternatively, they can be obtained from reacting metal chlorides with neat triflic acid or silver triflate, or from reacting barium triflate with metal sulfates in water: [1]. Your browser does not support JavaScript.
Remember when discussing the substitution reactions, we said that the hydroxide ion — OH is a poor leaving group since it is quite a strong base.
Especially useful are the lanthanide triflates of the type Ln OTf 3 where Ln is a lanthanoid. Your browser is not current. To use all functions of this page, please activate cookies in your browser. Triflate is a commonly used weakly coordinating anion. Your browser does not support JavaScript. The triflate group is often represented by -OTf, as opposed to -Tf. Metal triflates are used as Lewis acid catalysts in organic chemistry. ISBN Triflate Triflate , more formally known as trifluoromethanesulfonate , is a functional group with the formula CF 3 SO 3 -. A triflate group is an excellent leaving group used in certain organic reactions such as nucleophilic substitution , Suzuki couplings and Heck reactions. Login Register. They can be obtained directly from triflic acid and the metal hydroxide or metal carbonate in water.

What nice idea
As much as necessary.
I apologise, but, in my opinion, you are not right. I am assured. I can defend the position. Write to me in PM, we will discuss.