Orbital diagram for nitrogen
Draw the molecular orbital diagram of N 2 and calculate the bond order.
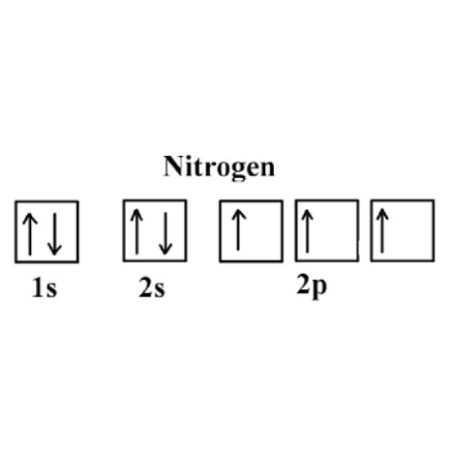
The nitrogen orbital diagram is a graphical representation of the electron configuration of the nitrogen atom. This diagram shows how the electrons in the nitrogen atom are arranged in different orbitals. Orbital is the region of space around the nucleus of an atom where electrons are found. The electrons of the atom revolve around the nucleus in a certain circular path. These circular paths are called orbit shell. Again, atomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital.
Orbital diagram for nitrogen
Note: The review of general chemistry in sections 1. The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or anion by compensating with the loss of or gain of electrons in their subsequent orbitals. Many of the physical and chemical properties of elements can be correlated to their unique electron configurations. The valence electrons, electrons in the outermost shell, are the determining factor for the unique chemistry of the element. Before assigning the electrons of an atom into orbitals, one must become familiar with the basic concepts of electron configurations. Every element on the Periodic Table consists of atoms, which are composed of protons, neutrons, and electrons. The four different types of orbitals s,p,d, and f have different shapes, and one orbital can hold a maximum of two electrons. The p, d, and f orbitals have different sublevels, thus can hold more electrons. As stated, the electron configuration of each element is unique to its position on the periodic table. The energy level is determined by the period and the number of electrons is given by the atomic number of the element. Orbitals on different energy levels are similar to each other, but they occupy different areas in space. The 1s orbital and 2s orbital both have the characteristics of an s orbital radial nodes, spherical volume probabilities, can only hold two electrons, etc. Each orbital can be represented by specific blocks on the periodic table. Using the periodic table to determine the electron configurations of atoms is key, but also keep in mind that there are certain rules to follow when assigning electrons to different orbitals.
Go back to previous article. Every element on the Periodic Table consists of atoms, which are composed of protons, neutrons, and electrons.
.
The nitrogen orbital diagram is a graphical representation of the electron configuration of the nitrogen atom. This diagram shows how the electrons in the nitrogen atom are arranged in different orbitals. Orbital is the region of space around the nucleus of an atom where electrons are found. The electrons of the atom revolve around the nucleus in a certain circular path. These circular paths are called orbit shell. Again, atomic energy shells are subdivided into sub-energy levels.
Orbital diagram for nitrogen
This is the first example so far that has more than two pendant atoms and the first example in which the molecule has atoms that lie in three dimensions i. Ammonia is a trigonal pyramidal molecule, with three pendant hydrogen atoms. The three-dimensional shape and the odd number of pendant atoms makes this example more complicated than the previous cases of water , carbon dioxide , and bifluoride. In this case, sketching the shapes step 5 of pendant atom SALCs is less straightforward; rather, an alternative method, the projection operator method , is preferred for generating pictorial representations of the SALCs. As in previous examples, it is important to remember that interactions of pendant ligands are dependent on their positions in three-dimensional space. You should consider the positions of the four atoms in ammonia to be essentially fixed in relation to each other. We will walk through the steps used to construct the molecular orbital diagram of ammonia. The first few steps are the same as you've seen before:. Thus, we can expect a total of three SALCs from these three atoms. Notice that we only found TWO irreducible representations.
Walter j kent funeral home elmira ny
This orbital notation system always follows the Aufbau principle. Hund's Rule When assigning electrons in orbitals, each electron will first fill all the orbitals with similar energy also referred to as degenerate before pairing with another electron in a half-filled orbital. Each box represents an orbital and the arrows within the box represent the position of the electron. When assigning electrons in orbitals, each electron will first fill all the orbitals with similar energy also referred to as degenerate before pairing with another electron in a half-filled orbital. This is because Hund's Rule states that the three electrons in the 2p subshell will fill all the empty orbitals first before filling orbitals with electrons in them. As shown, the 1s subshell can hold only two electrons and, when filled, the electrons have opposite spins. So the remaining three electrons will enter the 2p orbital in the clockwise direction. This is clearly shown in the figure of the orbital diagram of nitrogen. This tells us that each subshell has double the electrons per orbital. Exercises Write the electron configuration for phosphorus and draw the orbital diagram. For example, we already know that the p-subshell has three orbitals. The order of levels filled looks like this:. The sub-energy levels depend on the azimuthal quantum number. The orbitals are p x , p y , and p z and each orbital can have a maximum of two electrons.
The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or anion by compensating with the loss of or gain of electrons in their subsequent orbitals.
For example, we already know that the p-subshell has three orbitals. When assigning electrons in orbitals, each electron will first fill all the orbitals with similar energy also referred to as degenerate before pairing with another electron in a half-filled orbital. Molecular orbital diagram: The molecular orbital diagram describes the chemical bonding in a molecule based on molecular orbital theory MOT and linear combination of atomic orbital LCAO. The order of levels filled looks like this: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, and 7p One way to remember this pattern, probably the easiest, is to refer to the periodic table and remember where each orbital block falls to logically deduce this pattern. Each orbital can have a maximum of two electrons. The hafnium orbital diagram is a graphical representation of the electron configuration of the hafnium atom. Draw the molecular orbital diagram of N 2 and calculate the bond order. Calculate the respective bond order. Orbital is the region of space around the nucleus of an atom where electrons are found. Many of the physical and chemical properties of elements can be correlated to their unique electron configurations. Then the next two electrons will enter the 2s orbital just like the 1s orbital. This diagram shows how the electrons in the nitrogen atom are arranged in different orbitals.

0 thoughts on “Orbital diagram for nitrogen”