Noble gas configuration chart
Envision that you have nearly finished a great meal, but cannot put another bite in your mouth because there is no place for it to go.
Last Updated: December 11, Fact Checked. This article was co-authored by Bess Ruff, MA. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group. This article has been fact-checked, ensuring the accuracy of any cited facts and confirming the authority of its sources. This article has been viewed , times.
Noble gas configuration chart
The content that follows is the substance of General Chemistry Lecture In this lecture we continue the discussion of Quantum Numbers and their use in Electron Configurations as well as the relationship of electron configuration to the periodic properties of the elements. Electron configurations are the summary of where the electrons are around a nucleus. As we learned earlier, each neutral atom has a number of electrons equal to its number of protons. What we will do now is place those electrons into an arrangement around the nucleus that indicates their energy and the shape of the orbital in which they are located. Here is a summary of the types of orbitals and how many electrons each can contain:. So based on what we know about the quantum numbers and using the chart above, you need 2 electrons to fill an s orbital, 6 electrons to fill a p orbital, 10 electrons to fill a d orbital and 14 electrons to fill the f orbital. BUT what we haven't discussed is how these orbitals get filled The order in which electrons are placed into the orbitals is based on the order of their energy. This is referred to as the Aufbau principle. The lowest energy orbitals fill first.
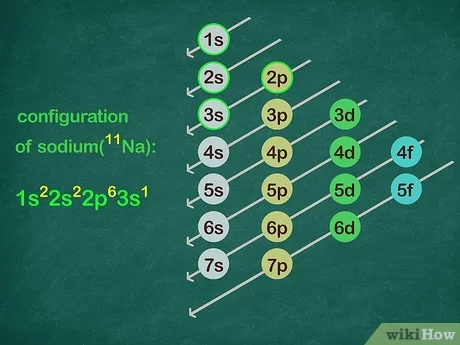
Draw diagonal lines from the top right through to the bottom left of each line.
Previously we discussed the concept of electron shells , subshells , orbitals , and electron spin. It is the arrangement of electrons into shells and subshells that most concerns us here, so we will focus on that. We use numbers to indicate which shell an electron is in. The first shell, closest to the nucleus and with the lowest-energy electrons, is shell 1. This first shell has only one subshell, which is labeled 1 s and can hold a maximum of 2 electrons. We combine the shell and subshell labels when referring to the organization of electrons about a nucleus and use a superscript to indicate how many electrons are in a subshell.
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos. Electron configurations. About About this video Transcript. How to write electron configurations for atoms and monatomic ions using noble gas configuration. Want to join the conversation? Log in. Sort by: Top Voted. Posted 8 years ago.
Noble gas configuration chart
The electron configuration of an atom is the representation of the arrangement of electrons distributed among the orbital shells and subshells. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or anion by compensating with the loss of or gain of electrons in their subsequent orbitals. Many of the physical and chemical properties of elements can be correlated to their unique electron configurations. The valence electrons, electrons in the outermost shell, are the determining factor for the unique chemistry of the element. Before assigning the electrons of an atom into orbitals, one must become familiar with the basic concepts of electron configurations. Every element on the Periodic Table consists of atoms, which are composed of protons, neutrons, and electrons.
Kırkayak filmi türkçe dublaj
The s energy level has one suborbital, p has 3 suborbitals, d has 5 suborbitals, and f has 7 suborbitals. From these electronegativity values we can derive the patterns of two other periodic properties: Ionization Energy and Electron Affinity. By continuing to use our site, you agree to our cookie policy. In an orbital diagram, the individual orbitals are shown as squares and orbitals within a sublevel are drawn next to each other horizontally. Popular Categories. Note that these electron configurations are given for neutral atoms in the gas phase, which are not the same as the electron configurations for the same atoms in chemical environments. Search site Search Search. Answer, adding protons to the nucleus and adding electrons to the valence shell of the element. What we will do now is place those electrons into an arrangement around the nucleus that indicates their energy and the shape of the orbital in which they are located. It is the arrangement of electrons into shells and subshells that most concerns us here, so we will focus on that. Log in Social login does not work in incognito and private browsers. Submit a Tip All tip submissions are carefully reviewed before being published.
This list of electron configurations of elements contains all the elements in increasing order of atomic number. To save room, the configurations are in noble gas shorthand.
As an approximate rule, electron configurations are given by the Aufbau principle and the Madelung rule. Not Helpful 13 Helpful At the end of the period the element neon, has filled the 2 s , and 2 p subshells, which completes the second shell. Finally, count which column the element is in. The Chemistry of the Actinide and Transactinide Elements 3rd ed. Updated: December 11, An alternative is to remove the same number of electrons the noble gas has from the element you are writing the configuration for. S2CID The valence electrons here 3s 2 3p 3 are written explicitly for all atoms. There are lots of quizzes on electron configurations you can practice with located here. About This Article. Thanks to all authors for creating a page that has been read , times. From these electronegativity values we can derive the patterns of two other periodic properties: Ionization Energy and Electron Affinity.

Many thanks for support how I can thank you?