Nh4 compound name
A cation is an electron-deficient species that carries a positive charge.
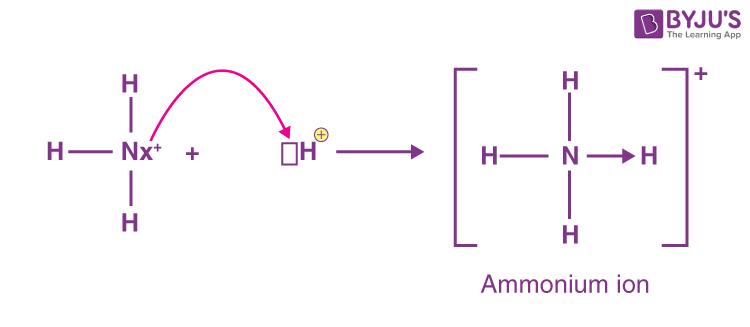
It is formed by the protonation of ammonia NH 3. Thus, the treatment of concentrated solutions of ammonium salts with a strong base gives ammonia. When ammonia is dissolved in water, a tiny amount of it converts to ammonium ions:. The degree to which ammonia forms the ammonium ion depends on the pH of the solution. If the pH is low, the equilibrium shifts to the right: more ammonia molecules are converted into ammonium ions. If the pH is high the concentration of hydrogen ions is low and hydroxide ions is high , the equilibrium shifts to the left: the hydroxide ion abstracts a proton from the ammonium ion, generating ammonia. Formation of ammonium compounds can also occur in the vapor phase; for example, when ammonia vapor comes in contact with hydrogen chloride vapor, a white cloud of ammonium chloride forms, which eventually settles out as a solid in a thin white layer on surfaces.
Nh4 compound name
.
It is formed by the protonation of ammonia NH 3. Primary, secondary, and tertiary ammonium salts serve the same function but are less lipophilic.
.
This is used when people wish to track nitrogen through the treatment process. Ammonia NH3 , shown in the middle, has a lone pair of electrons, and since nitrogen is more electronegative than hydrogen, the nitrogen atom has a partial negative charge red color. NH4 ammonium is a nontoxic salt, it is the ionised form of toxic ammonia NH3. It is useful to understand that in the aquatic environment NH4 is not toxic, however it does have the ability to instantly change to NH3 with a change in pH and or tempertaure. OH — is called a hydroxyl ion and it makes things basic. Pure water is neither acidic or basic; it is neutral. So how does something become acidic or basic? Ammonia is moderately basic; a 1. And all of them form an anion with a single negative charge. The VIA elements gain two electrons to form anions with a 2- charge….
Nh4 compound name
Ionic compounds are named using the formula unit and by following some important conventions. First, the name of the cation is written first followed by the name of the anion. Because most metals form cations and most nonmetals form anions, formulas typically list the metal first and then the nonmetal. Second, charges are not included in the name or the formula. Remember that in an ionic compound, the component species are ions, not neutral atoms, even though the formula does not contain charges.
Https account live com resetpassword aspx
February 3, Ammonium cation is found in a variety of salts such as ammonium carbonate , ammonium chloride , and ammonium nitrate. VIAS Encyclopedia. Category:Astrochemistry Outer space portal Astronomy portal Chemistry portal. In an unusual process, ammonium ions form an amalgam. Retrieved Watch Now. What is the formal charge on ammonium ion? To further confirm ammonia, it passed through a glass rod dipped in an HCl solution hydrochloric acid , creating white dense fumes of ammonium chloride. The ammonium salts of nitrate and especially perchlorate are highly explosive, in these cases, ammonium is the reducing agent. Bibcode : JChEd If the pH is high the concentration of hydrogen ions is low and hydroxide ions is high , the equilibrium shifts to the left: the hydroxide ion abstracts a proton from the ammonium ion, generating ammonia.
A cation is an electron-deficient species that carries a positive charge. A polyatomic ion, also known as a molecular ion, is a covalently bonded set of two or more atoms or a metal complex that can be thought of as a single unit and has a net charge that is greater than zero.
If the solution releases a gas with a characteristic smell when an alkali hydroxide is added to it and heated, it will confirm the presence of ammonium ions. Ammonium salts. Why is ammonium an ion? Ammonium is an important source of nitrogen for many plant species, especially those growing on hypoxic soils. Ammonium cation is found in a variety of salts such as ammonium carbonate , ammonium chloride , and ammonium nitrate. To find whether the ammonium ion is present in the salt, first, the salt is heated in presence of alkali hydroxide releasing a gas with a characteristic smell, which is ammonia. The ammonium salts of nitrate and especially perchlorate are highly explosive, in these cases, ammonium is the reducing agent. In mammals , sharks , and amphibians , it is converted in the urea cycle to urea , because urea is less toxic and can be stored more efficiently. Monthly Notices of the Royal Astronomical Society. Put your understanding of this concept to test by answering a few MCQs. Unsourced material may be challenged and removed. PubChem CID. Bibcode : Natur. If the pH is high the concentration of hydrogen ions is low and hydroxide ions is high , the equilibrium shifts to the left: the hydroxide ion abstracts a proton from the ammonium ion, generating ammonia. A polyatomic ion, also known as a molecular ion, is a covalently bonded set of two or more atoms or a metal complex that can be thought of as a single unit and has a net charge that is greater than zero.

I think, that you commit an error. I suggest it to discuss. Write to me in PM, we will talk.
In it something is. Now all is clear, I thank for the help in this question.