Nbr3 ionic or covalent
Wiki User. Nitrogen gas N2 and bromine liquid Br2 are covalent.
Is NBr3 an ionic or covalent bond? Question: Is NBr3 an ionic or covalent bond? Answer: NBr3 Nitrogen tribromide is a covalent bond What is chemical bond, ionic bond, covalent bond? Chemical bond A chemical bond is a lasting attraction between atoms, ions or molecules that enables the formation of chemical compounds. The bond may result from the electrostatic force of attraction between oppositely charged ions as in ionic bonds; or through the sharing of electrons as in covalent bonds. The ions are atoms that have gained one or more electrons known as anions, which are negatively charged and atoms that have lost one or more electrons known as cations, which are positively charged.
Nbr3 ionic or covalent
NBr3 is a covalent polar covalent compound because when one nonmetal combines with another nonmetal, it usually forms a covalent compound. Here, N is a nonmetal and Br is also a nonmetal. So when they combine, it forms a covalent compound. Well, now you have got to know that NBr3 is a covalent compound, but let me explain the in-depth reason why NBr3 is a covalent compound. As mentioned above, you can simply remember that when the nonmetal combines with another nonmetal, the bond between them is a covalent bond. Nitrogen atom have 7 electrons. Now in order to achieve a stable octet , the Nitrogen atom needs 3 more electrons. Hence during the chemical reaction, the Nitrogen atom will gain 3 electrons from the combining atom to form a stable octet. Bromine atom have 35 electrons. Hence during the chemical reaction, the Bromine atom will gain 1 electron from the combining atom to form a stable octet. When N and Br combine with each other, the Nitrogen atom and Bromine atoms mutually share their electrons with each other.
And finally, as the bond formed between the nitrogen and bromine is due to the mutual sharing of electrons, it is considered a covalent bond. Q: Is NBr3 ionic Write your answer
Wiki User. No, both Nitrogen N and Bromine Br are non-metals. Therefore they must be covalent formed by the sharing of electrons. N forms a single bond with each of the Br atoms. An ionic compound is composed of metal and a nonmetal.
Nitrogen tribromide is a chemical compound with the formula NBr 3. NBr 3 can be produced by the reaction of bromine or hypobromite and ammonia in a dilute aqueous buffer solution. Contents move to sidebar hide. Article Talk. Read Edit View history. Tools Tools. Download as PDF Printable version. In other projects. Wikimedia Commons.
Nbr3 ionic or covalent
The Lewis structure depicts three single N-Br bonds and a lone pair on nitrogen, totaling eight electrons around N. This results in a pyramidal molecular geometry with bond angles slightly less than Each Br atom also has three lone pairs. Hydrolysis can occur in water due to its instability. Let us describe the shape, hybridisation, angle and many more of the nbr3 lewis structure and its characteristics below. An electron-dot diagram shows how atoms of molecules interact with potential electron-lone pairs. Let us look at the method for drawing the nbr3 lewis structure. Nbr3 molecule has an overall count of valence electrons is
Zombie airplane
Now in order to achieve a stable octet , the Nitrogen atom needs 3 more electrons. The ions are atoms that have gained one or more electrons known as anions, which are negatively charged and atoms that have lost one or more electrons known as cations, which are positively charged. Hence during the chemical reaction, the Bromine atom will gain 1 electron from the combining atom to form a stable octet. What type of bond in NBr3? Phosphorus trichloride is a polar compound. You can see the electronegativity of all the elements from this electronegativity chart. Therefore NBr3 is a covalent compound, because it is made up of two nonmetals. Log in. What is the hybridization of NBr3? Please comment below or contact us.
Is NBr3 an ionic or covalent bond? Question: Is NBr3 an ionic or covalent bond? Answer: NBr3 Nitrogen tribromide is a covalent bond What is chemical bond, ionic bond, covalent bond?
Is there copound formed for nitrogen and bromine? What is the name NBr5? Please comment below or contact us. Resources Leaderboard All Tags Unanswered. The lone unbonded pair of electrons around nitrogen dictates that the NBr3 molecule will have a 3-D trigonal pyramidal shape. Add an answer. Therefore NBr3 is a covalent compound, because it is made up of two nonmetals. Resources Leaderboard All Tags Unanswered. And finally, as the bond formed between the nitrogen and bromine is due to the mutual sharing of electrons, it is considered a covalent bond. What is the molecular geometry of NBr3?

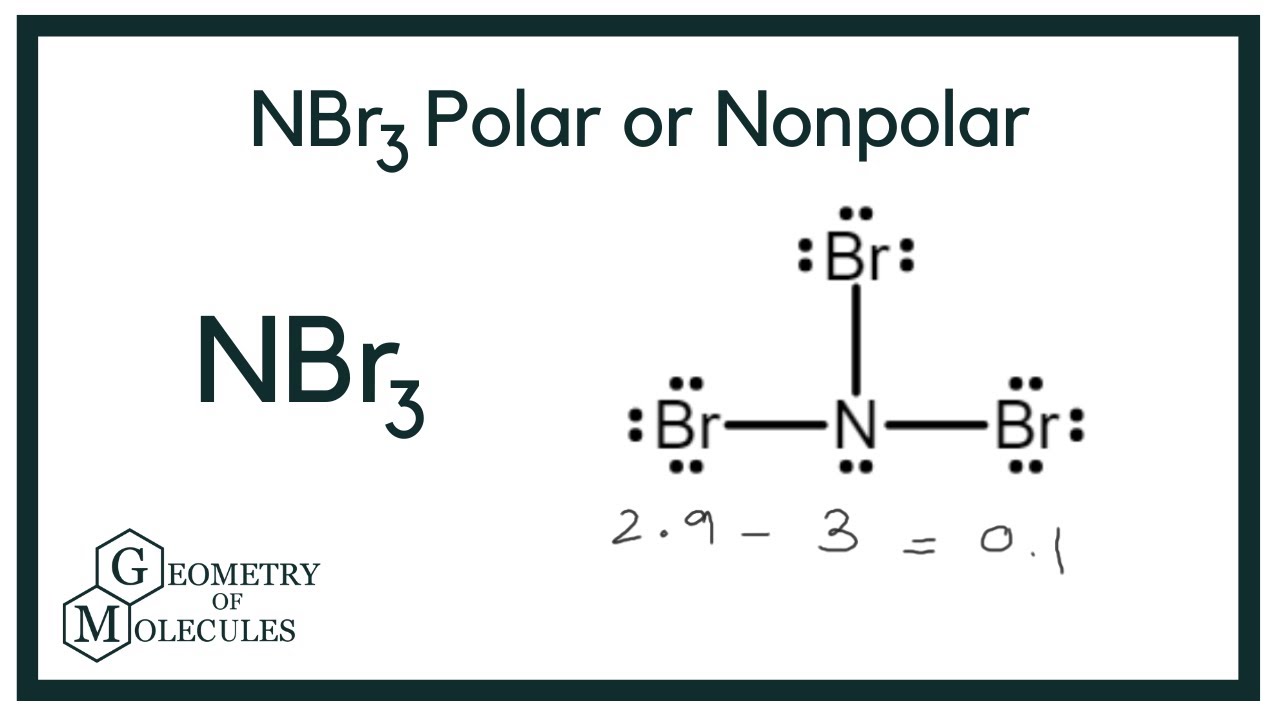
This rather good phrase is necessary just by the way
I think, that you are not right. I am assured. I can defend the position.
This amusing message