Nacl boiling point
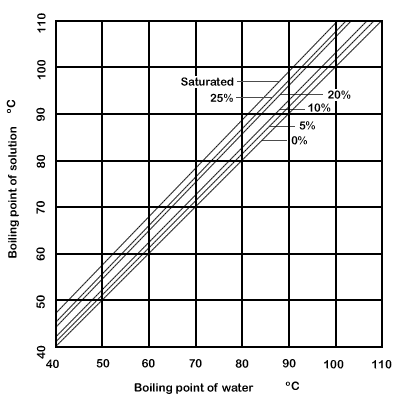
If you add salt to water, you raise the water's boiling point, or the temperature at which it will boil, nacl boiling point. The temperature needed to boil will increase about 0. This is an example of boiling point elevationand it is not exclusive to water. It occurs any time you add a nonvolatile solute such as salt to a solvent nacl boiling point as water.
See more Sodium products. Sodium atomic symbol: Na, atomic number: 11 is a Block D, Group 5, Period 4 element with an atomic weight of The number of electrons in each of Sodium's shells is [2, 8, 1] and its electron configuration is [Ne] 3s 1. The sodium atom has a radius of Sodium was discovered and first isolated by Sir Humphrey Davy in
Nacl boiling point
Solution A contains 1 mole of sodium chloride in 1 lite of wats? Solution B contains 1 mole of potassium chlorice in 1 lite of water. Both solutions are heated and the boiling point is measured. Which of the following statements is correct? A Solution A has a higher boiling point B Solution B has a higher boiling point C Both of solutions will have the same boiling point D More information is required to determine the relative boiling points. Pure water has a boiling point of however as you add salt to it, it makes it harder for the water molecules to escape from the pot and enter the gas phase. The more salt you add, the harder it is. So the boiling point increases with the concentration. Hope this helps!! Does this help? ChuHtet August 18, , pm 1. A Solution A has a higher boiling point B Solution B has a higher boiling point C Both of solutions will have the same boiling point D More information is required to determine the relative boiling points The answer is C.
Conversion Factors.
Skip to main content. Table of contents. Intro to General Chemistry 3h 53m. Classification of Matter. Chemical Properties. Physical Properties.
Many of the physical properties of solutions differ significantly from those of the pure substances discussed in earlier chapters, and these differences have important consequences. Aqueous solutions have both a lower freezing point and a higher boiling point than pure water. This solute lowers the freezing point of the water, preventing the engine from cracking in very cold weather from the expansion of pure water on freezing. Changes in the freezing point and boiling point of a solution depend primarily on the number of solute particles present rather than the kind of particles. As we will see, the vapor pressure and osmotic pressure of solutions are also colligative properties. When we determine the number of particles in a solution, it is important to remember that not all solutions with the same molarity contain the same concentration of solute particles. Consider, for example, 0.
Nacl boiling point
Sodium chloride is also known as salt. It occurs in oceans and sea waters. It is also found as rock salt. It is a crystalline solid , white. In its aqueous form, it is called a saline solution. This compound is water-soluble and consists of sodium cation and chloride anion.
Hangover download
Lewis Dot Structures: Ions. Hydrohalogenation Reactions. Average Rate of Reaction. Chemical Manufacturing. First Law of Thermodynamics -. Amide Formation. The Electron Configurations: Exceptions. Boron Family Reactions. When you add a solute that increases the amount of energy heat needed for water to make the transition, a few processes occur. The number of electrons in each of Sodium's shells is [2, 8, 1] and its electron configuration is [Ne] 3s 1. Product Number: All applicable American Elements product codes, e. Diprotic Acids and Bases. Quantum Numbers: Magnetic Quantum Number. Nuclear Binding Energy.
If you add salt to water, you raise the water's boiling point, or the temperature at which it will boil. The temperature needed to boil will increase about 0. This is an example of boiling point elevation , and it is not exclusive to water.
Sodium 11 Na The Colligative Properties. Structure and dynamics of cetyltrimethylammonium chloride-sodium dodecylsulfate CTAC-SDS catanionic vesicles: High-value nano-vehicles from low-cost surfactants. Solution Stoichiometry. When handling quantities of this material, be sure to wear appropriate protective equipment as described. Email Tweet Facebook. It is rarely found by itself in nature. Table of contents. Chuo University Researchers pioneer non-destructive inspection technique. Sodium does not occur in nature as a free element and must be extracted from its compounds e.

Amusing state of affairs
Unsuccessful idea