N2o lewis dot
Skip to main content. Table of contents.
Previously, we discussed how to write Lewis structures for molecules and polyatomic ions. In some cases, however, there is seemingly more than one valid structure for a molecule. We can use the concept of formal charges to help us predict the most appropriate Lewis structure when more than one is reasonable. The formal charge of an atom in a molecule is the hypothetical charge the atom would have if we could redistribute the electrons in the bonds evenly between the atoms. Another way of saying this is that formal charge results when we take the number of valence electrons of a neutral atom, subtract the nonbonding electrons, and then subtract the number of bonds connected to that atom in the Lewis structure.
N2o lewis dot
Post by » Mon Nov 05, am. Post by chaggard » Mon Nov 05, am. Post by » Tue Nov 06, am. Post by mbaker4E » Tue Nov 06, am. Post by Michael Nirula » Wed Nov 07, am. Laurence Lavelle Skip to content. Quick links. Email Link. Re: Lewis Structure for N2O Post by chaggard » Mon Nov 05, am N goes in the center because you want to have the lowest ionization energy element in the center. The structure has a triple bond to the other N atom, and a single on the O atom. Does it relate to the number of electrons filling the d-orbital? Nitrogen is placed in the center of the Lewis structure because it is the least electronegative, and since oxygen is more electronegative, its formal charge will have to be negative and therefore it is not placed in the center even though it has lower ionization energy. Nitrogen has a higher ionization energy because it has a half-filled 2p shell that makes it more stable unlike oxygen which has 2 unpaired electrons in its 2p shell which results in more electron repulsions and therefore lowers the ionization energy. Ionization energy represents the amount of energy required to remove a valence electron. Jump to.
Chemistry: Structure and Properties, 3rd ed. Vapor Pressure Lowering Raoult's Law. General rules for drawing Lewis structures.
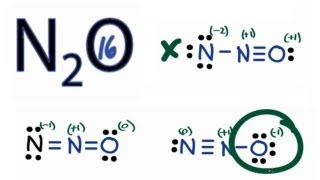
I learned this by the counting-electron method, and then assigning formal charges to determine the most likely distribution of valence electrons. We have two nitrogens and one oxygen, which suggests that either we have oxygen in the middle or two nitrogens in a row. Notice how if you had oxygen in the middle , the formal charges of both nitrogens have no way of being distributed well without exceeding 8 electrons for oxygen :. Now we can get two plausible possibilities, which are both linear molecular geometries NOT bent!!! Two electron groups! Truong-Son N.
The Oxygen atom has 3 lone pairs and the outer nitrogen atom has 1 lone pair. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of N2O. Here, the given molecule is N2O. In order to draw the lewis structure of N2O, first of all you have to find the total number of valence electrons present in the N2O molecule. Valence electrons are the number of electrons present in the outermost shell of an atom. Nitrogen is a group 15 element on the periodic table. Oxygen is a group 16 element on the periodic table. Remember: Fluorine is the most electronegative element on the periodic table and the electronegativity decreases as we move right to left in the periodic table as well as top to bottom in the periodic table. Here in the N2O molecule, if we compare the nitrogen atom N and oxygen atom O , then the nitrogen is less electronegative than oxygen.
N2o lewis dot
This article discusses N2O lewis structure and its hybridization, shape, bond angle, and relevant detailed explanations. N 2 O is covalent molecule. The central N atom is sp hybridized and terminal N and O are sp, and sp 3 hybridized respectively. Being sp hybridization the geometry of Nitrous Oxide is linear. So, the N-N-O bond angle is 0.
Airplane for sale canada kijiji
It is single bonded to two of these oxygen atoms, each of which has three lone pairs of electrons, and double bonded to the third, which has two lone pairs of electrons. Strong Titrate-Strong Titrant Curves. Thus, one of the nitrogens must be in the middle. Helium does not form stable covalent bonds. Molecular Formula. Naming Ethers. Lewis structures are preferable when adjacent formal charges are zero or of the opposite sign. Dipole Moment. Arrhenius Acids and Bases. There are 16 valence electrons in N 2 O. Textbook Question. Scientific Notation.
Transcript: Let's do the N2O Lewis structure. N2O has 16 total valence electrons. There's three ways we can draw it, and all of them work pretty well.
Periodic Trend: Successive Ionization Energies. The left structure depicts a carbon atom bonded to three oxygen atoms. Addition and Subtraction Operations. Lewis Dot Structures: Neutral Compounds. Related Videos. Such is the case for most organic molecules, those containing primarily carbon, hydrogen, and oxygen, with other elements such as nitrogen, sulfur, and phosphorous also common in molecules of biological importance. Henderson-Hasselbalch Equation. Test for Ions and Gases. Since either N or O can serve as a central atom, in choosing a skeletal structure we again use the guideline that the least electronegative atom be placed in the middle. From this point the electrons are moved if necessary in pairs in order that the octet rule is satisfied.

I consider, that you commit an error. Let's discuss. Write to me in PM.