Molecular shape of h2o2
In this post, we will be drawing the Lewis structure, and determining the geometry and hybridization of hydrogen peroxide, H 2 O 2. The first thing we need to do when drawing a Lewis structure is determine the total number of valence electrons in the molecule. Remember, molecular shape of h2o2, valence electrons are those in the outermost principal energy level.
It's well known that the structure of H2O2 is non-planar. Learn more about the structure of H2O2 and its meaning in detail! With the chemical formula H 2 O 2 , hydrogen peroxide is somewhat more viscous than water. In the presence of light, it is unstable and decomposable. It can also be present in the human body. Is H 2 O 2 nonpolar or polar, and so forth. H 2 O 2 is a colourless liquid with a bitter taste when it is pure.
Molecular shape of h2o2
.
Check this question multiple-choice quiz on Geometry and Hybridization: Free. Next, we check if the oxygens have octets, and two bonds with two lone pairs indeed satisfy the octet of each oxygen:.
.
In this post, we will be drawing the Lewis structure, and determining the geometry and hybridization of hydrogen peroxide, H 2 O 2. The first thing we need to do when drawing a Lewis structure is determine the total number of valence electrons in the molecule. Remember, valence electrons are those in the outermost principal energy level. For example: Na — 1s 2 2s 2 2p 6 3s 1 , Cl — 1s 2 2s 2 2p 6 3s 2 3p 5 The number of valence electrons, for main group elements, corresponds to their group number in the periodic table:. For example, iron has eight valence electrons: Fe — 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. So, oxygen is in group 6A, and therefore, it has 6 valence electrons, and hydrogen has one electron. Next, we need to connect the atoms in the correct order and add the electrons as bonds and lone pairs. In short, these are the steps you need to follow for drawing a Lewis structure :. Add the remaining electrons to satisfy the octet for a more electronegative atom first. If any atoms lack an octet, make a double or triple bond to give them an octet.
Molecular shape of h2o2
It is the simplest peroxide compound, i. It is a pale blue liquid in its standard state and slowly reacts with sunlight and decomposes into water and oxygen. H2O2 has a melting point of A low melting point indicates the tendency of the compound to remain in the liquid state. It also exhibits a relatively high boiling point of This is an example of a comproportionation reaction, i. It is a strong oxidizing agent, and hence, it finds wide applications as a bleaching agent and disinfectant. It is also used as an oxidizer in spacecraft since oxygen is not available for combustion in outer space.
Travel cameras oregon
Make a single link between hydrogen and oxygen. Enthalpy of Neutralisation. Steps in the Ring Closure. Get subscription. In the H2O2 structure, hydrogen already shares two electrons with the help of a single bond with an oxygen atom. JEE Main Highlights. As a result, the remaining valence electrons were arranged around oxygen to complete the octet rule. Hydrogen Peroxide is used to disinfect tools, bleach hair, and clean shells. How does hydrogen peroxide H2O2 work as a bleaching agent? Conclusion H2O2, a colourless liquid created as a solution of many strengths, is primarily used for bleaching cotton and other textiles, wood pulp, synthesis of other chemicals, as rocket fuel, and cosmetic and therapeutic uses.
Hydrogen Peroxide or H2O2 is widely used as an oxidizing agent and as an antiseptic. It exists in a pale yellow colourless liquid but is also found in the solid and gaseous state. Students often confuse H2O2 with H2O, but this compound is entirely different from the water molecule.
Many people are unsure whether H 2 O 2 is polar or nonpolar. Related articles. Follow some steps to drawing the H 2 O 2 Lewis dot structure Determine the total number of valence electrons in H 2 O 2. Why is the molecular geometry of the H2O2 Lewis structure bent, but the electron geometry is tetrahedral? Thus, hydrogen The first thing we need to do when drawing a Lewis structure is determine the total number of valence electrons in the molecule. Ans : Because each oxygen in the structure of H2O2 has an Sp3 hybrid that adopts a tetrahedr So, it primarily removes the harmful stuff, but it also kills some of the beneficial effects that your body requires to heal properly. The molecular geometry of hydrogen peroxide H 2 O 2 is a non-planar molecule and is even said to have an open book structure. JEE Eligibility Criteria

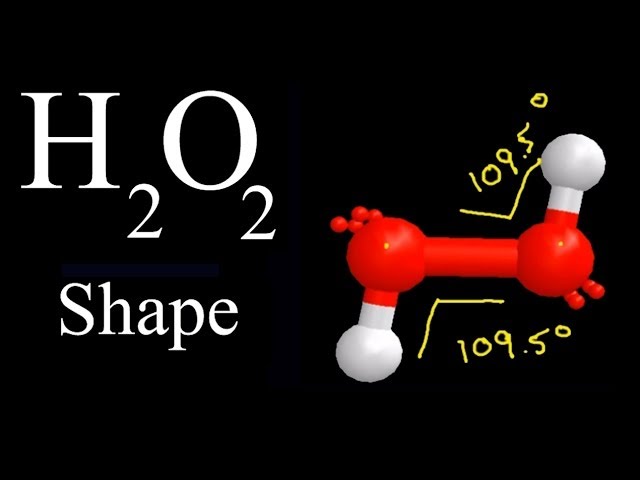
0 thoughts on “Molecular shape of h2o2”