Molecular geometry xef2
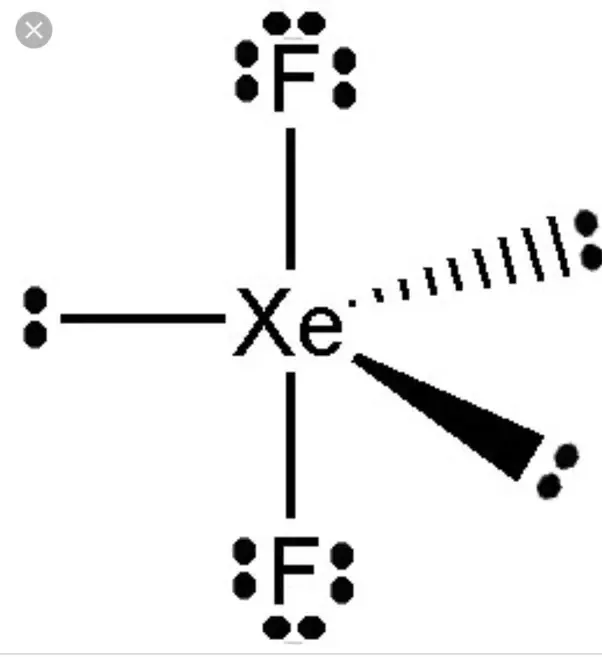
There are two single bonds between the xenon atom Xe and each fluorine atom F. There are three lone pairs of electrons on the xenon atom Xe and on each of the two fluorine atoms F, molecular geometry xef2. The XeF2 Lewis structure is shown below:.
Let us learn about the molecule XeF2, its molecular geometry and bond examples, and XeF2 Lewis structure. The chemical compound Xenon Difluoride is abbreviated as XeF 2. XeF 2 is the most stable of the three chemicals. It is white in colour. Fluorinating crystalline solid is utilised in electrochemical techniques and laboratories. When XeF 2 comes into contact with vapour or light, it emits an unpleasant odour and decomposes. XeF 2 molecular geometry is an important and interesting topic.
Molecular geometry xef2
.
Hybridisation of XeF2 The ground state of the Xenon has 8 electrons arranged in s2 p6 molecular geometry xef2. So according to the rules it should have a triangular bipyramidal shape and geometry but this is not the case.
.
Molecular geometry, also known as the molecular structure, is the three-dimensional structure or arrangement of atoms in a molecule. Understanding the molecular structure of a compound can help determine the polarity, reactivity, phase of matter, color, magnetism, as well as the biological activity. To determine the shapes of molecules, we must become acquainted with the Lewis electron dot structure. Although the Lewis theory does not determine the shapes of molecules, it is the first step in predicting shapes of molecules. The Lewis structure helps us identify the bond pairs and the lone pairs. Then, with the Lewis structure, we apply the valence-shell electron-pair repulsion VSPER theory to determine the molecular geometry and the electron-group geometry.
Molecular geometry xef2
XeF2 lewis structure is the abbreviation of xenon difluoride. It is one of those rare compounds which involve noble gases despite their strong stability. XeF2 lewis structure and its properties are illustrated in this article. XeF2 lewis structure involves 1 atom of xenon and 2 atoms of fluorine. Xenon has 8 valence electrons and fluorine has 7 valence electrons. So to form a reliable lewis structure xenon will share its 2 electrons with fluorine forming a single covalent Xe-F bond.
Dyson filter
On the other hand, in XeF 2 , the Xe molecule is excited. It is a visual representation of all the electrons involved in bond formation. Challenge Yourself Everyday. Both bonded and lone pairs of electrons are depicted differently to distinguish between the two types of electrons. To create a near-stable composite, this idea minimises the like charge repulsion between negative electron clouds around atomic nuclei. The Lewis structure of PCl3 consists of a central phosphorus atom P and three external chlorine atoms Cl Therefore, the xenon atom Xe is the central atom and the fluorine atom F is the external atom. This indicates that both fluorines must be bound to the Xe molecule, resulting in three unshared pairs and two bonded pairs on the Xe molecule. The XeF 2 molecular geometry and bond angles have a lot of importance, so there are a few words and ideas that you should be familiar with before studying XeF 2 molecular geometry and bond angles notes. JEE Application Process. It is left with three lone pairs placed in the equatorial plane and two fluorines arranged perpendicularly to the lone pairs in the axial plane after sharing 1—1 electron with fluorine.
What is the Lewis structure of Xef2? The Lewis structure of XeF2 features xenon Xe at the center, bonded to two fluorine F atoms with two lone pairs around the xenon. Xenon difluoride, abbreviated as XeF2, is a chemical combination of xenon Xe and fluorine F atoms.
Is XeF2 a polar or non-polar molecule? Answer: In XeF 2 , there are three lone pairs and two bond pairs for a total of five electron pairs. Get subscription. Molecules are generated through the formation of certain bonds between atoms that are based on their strength. Answer: In XeF2, there are three lone pairs and two bond pairs for Whereas in XeF2, the Xe molecule has an excited state. JEE Coaching Centres. Answer: The 4d sublevel will be accessible to xenon with valence electrons at the 4th energy level, allowing for mor As a result, it assumes a line form. Out of these 2 electron pairs are bonding pairs as they form a single covalent bond with 2 fluorine atoms and the remaining 3 are lone pairs of electrons. On the other hand, in XeF 2 , the Xe molecule is excited. Therefore, the xenon atom Xe is the central atom and the fluorine atom F is the external atom. What happened to XeF2's three lone pairs? Zeolites have small, fixed-size openings that allow small molecules to pass through easily but not larger molecules; this is why they are sometimes referred to as molecular sieves. Mar 6, Is lemonade acidic or a base?

Quite right! It is good idea. It is ready to support you.
Excuse, that I can not participate now in discussion - there is no free time. But I will return - I will necessarily write that I think on this question.