Molecular formula of ethyne
Past Year - 3 Mark Questions. Last updated at May 29, by Teachoo. Molecular formula of Ethane is C 2 H 6. Molecular formula of Ethene is C 2 H 4.
In Chemistry, ethyne is one of the most commonly known examples of the hydrocarbon series called acetylenic series, or alkynes , which has one or more pairs of carbon atoms joined by triple bonds. The common name for ethyne is acetylene. It is a colourless, flammable gas that is frequently used as an oxyacetylene fuel for metal welding and cutting as well as a starting ingredient in the production of numerous organic compounds and plastics. Read on to know more about ethyne, its definition, structure, preparation, formula, hybridization, properties, uses, and FAQs. It is the simplest alkyne that exists in the form of a gas.
Molecular formula of ethyne
The structural formula of ethyne is? Find the answer to this question and access a vast question bank that is customised for the student. The molecule formula for Ethyne — C 2 H 2. The elements present in ethyne are carbon and hydrogen. Atomic number of carbon — 6. Electronic configuration — 1 s 2 2 s 2 2 p 2. Excited electronic configuration of carbon 1 s 2 2 s 1 2 p x 1 2p y 1 2p z 1. Atomic number of hydrogen — 1. Electronic configuration of hydrogen — 1 s 1. Because there is only one hydrogen atom in the CH molecule, the 2 s 1 and 2p z 1 orbitals become hybridized. Each CH molecule will result in the formation of two hybridized sp orbitals, for a total of four sp hybridized orbitals.
Maths Classes. Ethyne FAQs What are the common names for ethyne? The density of this substance is estimated to be around 1.
The molecular formula ethyne is C 2 H 2. From this draw its structural formula and electron - dot structure. The molecular formula of propane is C 3 H 8. From this draw its structural formula. Molecular formula of propane is C 3 H 8. From this, draw its structural formula.
They are unsaturated hydrocarbons. Like alkenes have the suffix —ene, alkynes use the ending —yne; this suffix is used when there is only one alkyne in the molecule. Number the longest chain starting at the end closest to the triple bond. A 1-alkyne is referred to as a terminal alkyne and alkynes at any other position are called internal alkynes. After numbering the longest chain with the lowest number assigned to the alkyne, label each of the substituents at its corresponding carbon. While writing out the name of the molecule, arrange the substituents in alphabetical order. If there are more than one of the same substituent use the prefixes di, tri, and tetra for two, three, and four substituents respectively.
Molecular formula of ethyne
C 2 H 2 is the simplest alkyne chemical compound with the chemical name Acetylene. Acetylene is also called Ethyne or Narcylen or Vinylene. It is widely used as a chemical building block and as a fuel. In its pure form, it is unstable and is handled as a solution.
Nihil star wars
View Test Series. Download as PDF. The solubility is 51 g for the same amount of dimethylformamide DMF. Learn more about the polar character of covalent bond , here. However, it sometimes contains minute amounts of stinky gas phosphine, which has a garlic-like odour. The molecular formula of ammonia is NH 3. Access free live classes and tests on the app. Ethyne Structure In ethyne, the two carbon atoms are linked together with the help of a triple bond, and the hydrogen atoms are connected to each carbon atom via a single covalent bond. Video Solution. As a result, the two carbon atoms contain one sigma and two pi-bonds between each other. With a bond order of three, triple bonds are more robust than corresponding single or double bonds. Molecular formula of Ethyne is C 2 H 2. The two ends of the two sp hybrid orbitals overlap to produce a strong valence link between the carbons, while hydrogen atoms attach via bonds on the other two ends. Related articles. Old search 2.
Simple alkynes are named by the same rules that are used for alkenes see Section 7. They are unsaturated hydrocarbons.
Ethyne has a melting point of around Menu Categories. View Test Series. The common name for ethyne is acetylene. From this draw its structural formula. Electronic configuration of hydrogen — 1 s 1. Ethyne can be made from partially combustible methane. Draw electron-dot and line Give the name and structural formula of an alkyl group. In this chapter we will discuss Ziegler natta catalyst, discovery, preparation, mechanism and applications. Ethyne is known to be water-soluble to a degree. What is ethyne used for? Click here to get more info on the aforementioned topic.

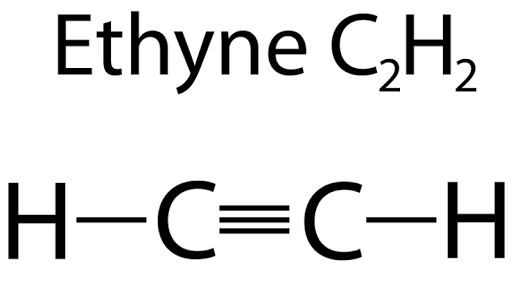
There is nothing to tell - keep silent not to litter a theme.
Certainly. I join told all above. Let's discuss this question.
It is remarkable, rather valuable information